Theoretical study on the structural, electronic and physical properties of layered alkaline-earth-group-4 transition-metal nitrides AEMN2
Abstract
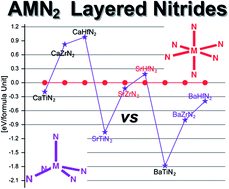
Thermodynamic, structural, and electronic properties of the layered ternary nitrides AEMN2 (AE = alkaline-earth; M = group 4 transition metal) both with the KCoO2 and α-NaFeO2 structure-types are examined within density-functional theory. The AE:M atomic (or ionic) radius ratio seems to be the most important criterion in determining one structural arrangement over the other. We find that the majority of compounds are more stable with the KCoO2 structure-type where M is coordinated to five nitrogen atoms in a distorted square-based pyramidal geometry. Strong interactions occur in both arrangements not only between nitrogen and transition metal atoms, but also between nitrogen and alkaline-earth metal atoms within and between the layers. Calculations show that all the AEMN2 compounds with the tetragonal structure-type KCoO2 are semiconducting with band gaps of approximately 1 eV. However, small band gap conductor and even semi-metallic behavior are computed for compounds with the alternative hexagonal α-NaFeO2 structure-type.

 Please wait while we load your content...
Please wait while we load your content...