Hierarchically structured PMMA fibers fabricated by electrospinning
Lu Li,
Zhao Jiang,
Mengmeng Li,
Ruosong Li and
Tao Fang*
Department of Chemical Engineering, Xi'an Jiaotong University, Xi'an 710049, China. E-mail: taofang@mail.xjtu.edu.cn
First published on 24th September 2014
Abstract
The role of solvents is pivotal for determining the primary and hierarchical structures of electrospun fibers. The preparation of beads-on-string structures or uniform poly methyl methacrylate (PMMA) fibers with circular and collapsed ribbon-like cross sections from six solvents with different properties was described in this work. The formation of a fiber cross section can be explained by buckling instability. Moreover, all of the resultant fiber surfaces are porous or wrinkled when electrospun with these solvents. It was concluded that vapor-induced phase separation (VIPS) by high relative humidity is the mechanism responsible for the formation of hierarchical structures. The nucleation growth (NG) mechanism during phase separation accounted for the round nanopores with radii of 60–150 nm and elliptical nanopores with long axis of 60–140 nm, short axis of 20–40 nm on the electrospun PMMA fibers from dichloromethane (DCM), chloroform, and ethyl acetate, whereas the formation of the wrinkled fiber surface of the PMMA/acetone, PMMA/tetrahydrofuran (THF), and PMMA/N,N-dimethyl formamide (DMF) systems resulted from spinodal decomposition (SD) mechanism. Furthermore, the fibers with round cross sections and highly porous interiors and surfaces were observed due to the VIPS and phase separation caused by the different evaporation rates of DCM and DMF.
1. Introduction
In recent years, one-dimensional micro-/nano-materials, micro-/nano-fibers have attracted broad attention and been widely used in many fields due to their fascinating and amazing characteristics such as high specific surface area, flexibility in surface functionalities, and superior mechanical performance.1 Nanofibers have been manufactured via a number of elegant fabrication approaches, including self-assembly, phase separation, drawing, template synthesis, and electrospinning.1–3 It is noteworthy that electrospinning, which has several fascinating and extraordinary features, including simple operation, controllable fiber diameter, and high porosity, is the most prominent, effective, and versatile approach for the fabrication of uniform fibers with micrometer- and nanometer-sized diameters in a continuous process. Formalas4 first published a patent about electrospinning describing an experimental setup that stretched viscous liquids into fibers using electrostatic forces. A variety of factors, such as electrospinning operation parameters (such as applied voltage, feed rate, tip to collector distance (TCD)), solution properties (such as solution viscosity, solution surface tension, solvent type, polymer molecular weight), and environmental conditions (such as temperature and relative humidity) have been exploited to control the morphologies and tailor the properties of electrospun fibers. As a result, beads, beads-on-string structure, cylinder fibers, flat ribbon-like fibers, and branched fibers, known as primary structures have been obtained in various studies.5–7 In addition to the well-studied primary structures, electrospun fibers exhibit numerous hierarchical structures, including a porous surface or core, wrinkled surface, and core–shell, necklace-like, hollow structures, in contrast to the smooth fibers traditionally generated by electrospinning. These hierarchical structures impart a huge specific area and other advantageous properties to the fibers, which make the fibers with hierarchical structures competitive candidates for applications in filtration and adsorption,8 catalysis,9,10 energy storage,11 and tissue engineering.12 Therefore, the preparation of fibers with hierarchical structures has been a subject of considerable interest.Different research has demonstrated that the formation of hierarchical structures on fibers could be directly obtained with high vapor pressure solvents13–15 or by using additives in solution, followed by removal in post-treatment, such as solvent extraction,16,17 thermal treatment,18 and light irradiation.19,20 In general, the method by selective removal of a sacrificial component requires an extra treatment after electrospinning, and the fibrous structures easily collapse or deform, resulting in the severe conditions with high-temperature calcination and longtime extraction.21 Moreover, some modifications of experimental electrospinning setup have been carried out for producing fibers with hierarchical structures. Shen et al.22 developed and patented23 a vessel in which the polymer solution could be electrospun in a near-critical CO2. Nanofibers with porous internal structure and smooth skin, or porous core and surface were obtained using this device.24,25 Nayani et al.26 modified the collector in a bath filled with water to induce porosity both in the core and on the surface of the electrospun poly acrylonitrile (PAN) fibers. However, the complexity, particular requirement and high cost of the electrospinning device hinder its further application. It is a reasonable speculation that the method based on highly volatile solvent should be most straightforward and versatile to form secondary structures on fibers during electrospinning. It is well documented that a volatile solvent plays a critical role in generation of pores and wrinkles on fiber surfaces.27–29 Numerous polymers, such as polystyrene (PS),27 poly-l-lactide (PLLA),13 polycarbonate (PC),13 ethyl cellulose,30 and cellulose triacetate,31 have been exploited to be directly electrospun into ultrafine fibers with porous or wrinkled surface morphologies. Furthermore, ternary systems containing both highly volatile and less volatile solvents have been proposed to create porosity via electrospinning.29,32 Finally, environmental conditions, i.e. relative humidity and temperature, which were largely neglected in past research, have been investigated as instrumental parameters for fabricating hierarchical structures both internally and externally.15,33,34
The formation of hierarchical structures throughout fibers is complex but will be advantageous both in an understanding of the process and facilitate the application of electrospinning. The formation mechanism of hierarchical structures has been contributed to two different mechanisms of phase separation32 and “Breath Figure”.35 The solvent evaporates during electrospinning and the resulting temperature decreases, leading to an instability of the solution, followed by the occurance of thermally induced phase separation (TIPS). When electrospun at high relative humidity, the solvent evaporates together with water and diffuses into polymer jets, resulting in vapor-induced phase separation (VIPS). In addition, because of the water condensation from the air and subsequent evaporation of water vapor and solvent, characteristic patterns generate on the fiber, known as “Breath Figure.” Zheng et al.15 explained that “Breath Figure” accounts for porous fibers electrospun with tetrahydrofuran (THF), whereas VIPS is the most pertinent mechanism of PS fibers with porous internal structure when electrospun with DMF. Pai et al.36 and Lu et al.21 elucidated that the VIPS is responsible for porous fibers obtained by electrospinning under high relative humidity. However, Dayal et al.37,38 theoretically investigated the electrospinning of porous PMMA fibers and suggested that TIPS is the reason for pores on fiber surfaces.
As previously mentioned, the exact mechanism of the formation of hierarchical structures in electrospun fibers is still unsettled. In this work, PMMA, a rigid polymer biomaterial with good compatibility with human tissue has been electrospun with several solvents under the temperature of 298 ± 2 K and a relative humidity of 38% ± 2%. Solvent plays an important role in the construction of both primary and hierarchical structures in electrospinning. Dichloromethane (DCM), acetone, chloroform, THF, ethyl acetate, and dimethyl formamide (DMF) with different properties were used to dissolve PMMA for the formation of fibers via electrospinning. The goal of this research is to investigate the effect of solvents on primary and hierarchical structures of PMMA fibers. Another objective of this study is to elucidate the exact mechanism responsible for the hierarchical structures and the flat, ribbon-like structure of the PMMA fibers.
2. Materials and method
2.1 Materials
Poly methyl methacrylate (PMMA, MW = 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) was purchased from Mitsubishi Rayon Polymer Nantong Co. Ltd. Dichloromethane (DCM), acetone, chloroform, tetrahydrofuran (THF), ethyl acetate, and N,N-dimethyl formamide (DMF) were purchased from Sinopharm Chemical Reagent Co. Ltd.
000 g mol−1) was purchased from Mitsubishi Rayon Polymer Nantong Co. Ltd. Dichloromethane (DCM), acetone, chloroform, tetrahydrofuran (THF), ethyl acetate, and N,N-dimethyl formamide (DMF) were purchased from Sinopharm Chemical Reagent Co. Ltd.
2.2 Electrospinning process
The PMMA was dissolved in DCM, acetone, ethyl acetate, chloroform, THF, and DMF and stirred sufficiently by a magnetic stirring apparatus at ambient temperature for 6 h. A self-assembled electrospinning apparatus consisted of a high-voltage power supply (BGG DC high-voltage generator) purchased from the BMEI Co. Ltd. (Beijing, China) and a digitally controlled syringe pump purchased from Longer Pump Baoding Co. Ltd., was introduced to produce PMMA fibers. The PMMA/solvent systems were placed in a glass syringe (10 mL) collected with the pump, which was used to supply a steady flow of solution to the tip of the needle. The electrospun fibers were collected on a piece of aluminium foil placed at a given distance. All of the experiments were carried out at 298 ± 2 K with a relative humidity of 38% ± 2%.2.3 Characterization
The viscosity of the PMMA solutions was measured at room temperature by NDJ-79 rotational viscometer, which was made by Shanghai Changji Instruments Co. Ltd. The conductivity of the solution was measured by DDS-11A conductivity, which was fabricated by Shanghai REX Instrument Factory. Three samples were tested for each system to ensure the reproducibility of the experimental results. The morphological appearance of the electrospun PMMA fibers was investigated visually with a scanning electron microscope (SEM), model JSM-6390. Each sample was coated with gold for 90 s prior to being observed under the SEM. The average diameter of the PMMA fibers was determined by analyzing the SEM images by Image Pro Plus 6.0 (Media Cybernetics, USA).3. Results and discussion
Solvent property is the key factor that influences the primary and hierarchical structures during electrospinning. Selecting an appropriate solvent is important to the morphologies, which would determine the application of the fibers. Six solvents with different volatilities are utilized to investigate the structure of electrospun fibers, and their physical properties are listed in Table 1. We have also performed some preliminary studies on the influences of electrospinning parameters and solution properties on the primary structure of the electrospun PMMA fiber.39 PMMA was dissolved in these solvents with a concentration of 20 wt%. Subsequently, these solutions were electrospun to investigate the influence of solvent on the fiber structures.| Solvent | Boiling point (K) | Density (g m−3) | Conductivity (S m−1) | Dielectric constant (293 K) | Surface tension (293 K, 10−3 N m−1) |
|---|---|---|---|---|---|
| DCM | 313 | 1.326 | 4.3 × 10−11 | 9.1 | 28.1 |
| Acetone | 329 | 0.790 | 5.0 × 10−9 | 20.6 | 23.3 |
| Chloroform | 334 | 1.480 | <1.0 × 10−10 | 4.8 | 27.2 |
| THF | 339 | 0.888 | 4.5 × 10−5 | 7.6 | 28.0 |
| Ethyl acetate | 350 | 0.895 | 1.0 × 10−9 | 6.0 | 24.0 |
| DMF | 426 | 0.945 | 6.0 × 10−8 | 36.7 | 35.0 |
3.1 Electrospinning with different solvents
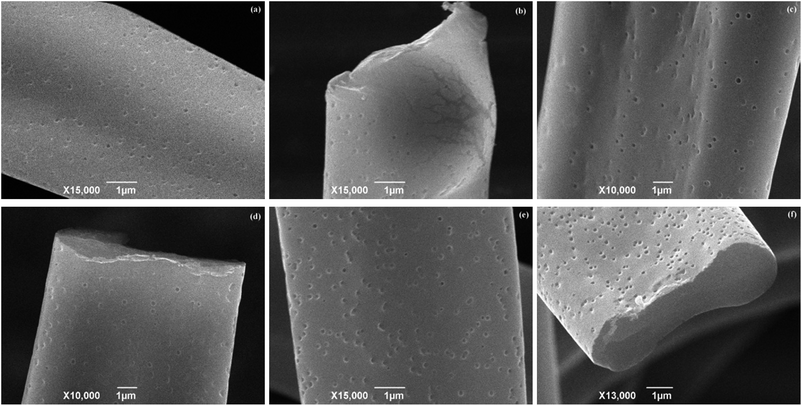
The porous PMMA fibers were fabricated by electrospinning a 20% PMMA/DCM solution under a set of process parameters: feed rate (1.2 mL h−1) and TCD (10 cm). The resultant PMMA fibers were examined by SEM, and the morphologies of the fibers are shown in Fig. 1. It is depicted that all the PMMA fiber mats have good uniformity without beads. All of the fibers obtained from different applied voltages have a collapsed ribbon-like structure with average width (fiber diameter) of 7.67 ± 0.78 μm, 5.22 ± 0.52 μm, 5.72 ± 0.82 μm, at 12 kV, 15 kV, and 18 kV, respectively. Moreover, it is noticed that there are isolated circular shape nanopores with diameter measured in narrow range of 60 nm to 100 nm that appear at the surface of electrospun PMMA fibers, as exhibited in Fig. 1(b, e and h). The pores on the fiber surface are all circular without changes in size when the applied voltage varied from 12 to 18 kV. The cross-sectional images of PMMA fibers (Fig. 1(c, f and i)) suggest that the nanopores are shallow and are not connected internally with each other, and the fibers are all with solid cores, which is different from the early research on PMMA.37 PMMA fibers with porous surfaces and interior interconnected elliptical pore structures were manufactured by electrospinning isotactic PMMA in DCM,37,38 which indicated that thermally induced phase separation caused by solvent evaporation can account for the formation of porous structure. | ||
| Fig. 1 SEM images of PMMA fibers electrospun from 20% PMMA/DCM solution-operated parameters: feed rate (1.2 mL h−1), TCD (10 cm), applied voltage: (a–c) 12 kV; (d–f) 15 kV; and (g–i) 18 kV. | ||
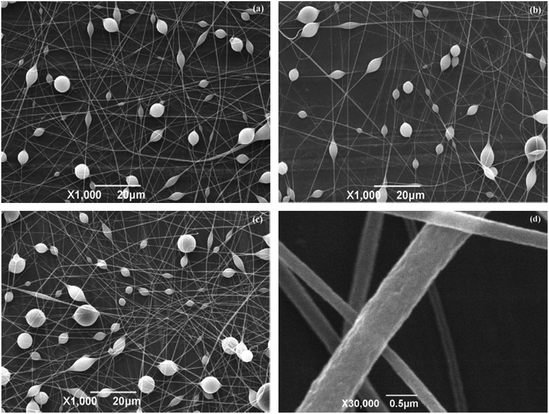
Fig. 2 represents the SEM images of PMMA fibers electrospun from 20% PMMA/acetone solution at different levels of applied voltage. It is shown that the fiber mats are all with beads, in which both the fibers and beads exhibit a wrinkled or collapsed surface. The fiber diameter of the beads-on-string structure is difficult to estimate in this system. The formation of beads and wrinkled fiber surfaces in electrospinning has been widely reported in a number of studies.28,41 As the applied voltage increased, the wrinkled surface of the fiber mat was not remarkably changed, and the beads did not disappear. It is reasonable to postulate that the Rayleigh instability of fluid jets, resulting from a low solution concentration of 20% was the dominant reason for the beads on the PMMA fibers. Piperno et al.41 have demonstrated that the beads disappear with the increase of concentration of PMMA to acetone. The wrinkled surfaces of beads and fibers are different with the porous fiber surface from the PMMA/DCM system. However, it is qualitatively similar to the result of Zheng's study,15 in which the wrinkled surface of PS fibers with the beads-on-string structure was observed due to the VIPS by the high relative humidity.
Chloroform with higher volatility than DCM and acetone was used to electrospin PMMA fibers. Keeping other conditions constant, 20% PMMA/chloroform solution was electrospun onto the fibers, and the SEM of PMMA fibers are exhibited in Fig. 3. It is shown that the resultant PMMA fiber mats are all without any beads, but with the presence of both large and small fibers. The average fiber is 4.46 ± 1.31 μm, 3.45 ± 1.35 μm, 2.94 ± 1.42 μm at 12 kV, 15 kV, and 18 kV, respectively. When the applied voltage is increased to 18 kV, there are a large number of thin fibers appearing with a diameter about 1.2 μm, indicated by the white arrow in Fig. 3(e). The increase in the voltage generally causes instability of a vibrating Taylor cone, resulting in uncontrolled scattering of fibers and the coexistence of large and tiny fibers. The cross section of a broken fiber (Fig. 3(b)) suggests that the fibers electrospun from the PMMA/chloroform system have collapsed, ribbon-like structures. The high-magnification SEM obviously shows that elliptical nanopores could be constructed on the fiber surface, whereas the pores on fibers from PMMA/DCM system are circular in shape. The short axis diameter distribution of the nanopores is from 25 nm to 40 nm, and the long axis diameter distribution is from 40 nm to 65 nm. Furthermore, the elliptical nanopores were aligned along the axis of the fiber, which is ascribed to the stretching from the electrical field during electrospinning.21 Compared to chloroform with lower volatility, DCM is an extremely volatile solvent. In electrospinning, DCM volatilizes so rapidly that the circle pores have insufficient time to stretch into elliptical shape by electrical force.
Fig. 4 shows the SEM images of the PMMA fibers electrospun from 20% PMMA/THF solution with a feed rate of 1.2 mL h−1 and TCD of 10 cm. The fiber mats reveal good uniformity with continuous fibers, as shown in Fig. 4(a, c and e). Both the average diameter and the diameter distribution of electrospun fibers increased with rising applied voltage, which is 1.73 ± 0.27 μm, 1.98 ± 0.36 μm, 2.08 ± 0.50 μm at 12 kV, 15 kV, and 18 kV, respectively. Compared to low voltage, the high acceleration by increasing voltage leads to less stretching in the electrical field, which makes the fiber diameter thicker. Simultaneously, the instability of solution jets under high voltage results in large fiber diameter distribution. Fig. 4(b, d and f) reveals that the fibers have wrinkled surfaces and cylindrical shapes and show aggravation of the wrinkled surface when compared to the fibers elecrospun from acetone. However, collapsed, ribbon-like PMMA fibers with smooth surfaces were produced when electrospinning with low molecular weight PMMA and THF by Qian et al.42 It is likely that the different polymer influences the solution properties, such as conductivity, viscosity, and surface tension, resulting in different fiber morphologies even with the same PMMA/THF system.
In our previous study,39 the effects of electrospinning parameters on the fiber diameter of the PMMA/ethyl acetate system have been systematically investigated. It was observed that all fibers electrospun from ethyl acetate have non-circular cross sections with ribbon-like structures. Further investigation on the fiber morphology of this system was conducted in the current study. High-magnification SEM image of electrospun PMMA fibers from 20% solution concentration is shown in Fig. 5. Uniform circular nanopores were observed in surface morphologies of samples produced with applied voltages 10 kV, 14 kV, and 18 kV, as shown in Fig. 5(a, c and f). The pore size has a wide distribution from 60 nm to 150 nm. The fiber cross-section in Fig. 5(b, d and e) shows that the pores are located predominantly on the surface, and the fibers have solid cores. In addition, the varied applied voltages have not significantly affected the fiber morphologies, pore sizes and number in both PMMA/DCM and PMMA/ethyl acetate systems. Compared to the PMMA/DCM and PMMA/chloroform systems, the pore number on the fibers is less when electrospun from ethyl acetate, which may be attributed to its low volatility.
We further studied the PMMA/DMF system because DMF has the highest vapor pressure and surface tension in this work. Fig. 6 shows the morphology of PMMA fiber electrospun with DMF. The poor spinnability of PMMA/DMF is clearly illustrated in Fig. 6, which exhibits the beads-on-string structure of PMMA fibers. In addition, with the increase of the applied voltage, beads on the fibers prepared from PMM/DMF showed an increase in number and no change in shape and structure. This observation reveals some similarity to the electrospinning of Poly (d,L-lactic acid) (PDLA) in DMF,43 which suggests that the increase of jet velocity and the decrease of droplet volume induced by increased voltage make the Taylor cone shape oscillate and become asymmetrical. Eventually, beads were prone to form with high voltage, and the surface morphology of the fibers was examined, as shown in Fig. 6(d). The high-magnification SEM visibly depicts the unsmooth wrinkled surface of the PMMA fibers. Because of DMF's miscibility with water and the hydrophobicity of PMMA, water vapor from the air diffuses into the streaming jet and induces the initially homogeneous polymer solution to become thermodynamically unstable, and phase separation then occurs. The PMMA-rich phase formed a solid fiber matrix, and the DMF-rich phase left wrinkles by solvent leaching.
A binary solvent system, DCM (high volatility) and DMF (low volatility), was used for electrospinning and studied in this work. Previous literature has demonstrated porous fiber formation with a binary solvent system.15,35 The samples were prepared from 20% PMMA solution with the DCM/DMF volume ratio of 3/1, and the SEM images of these samples are displayed in Fig. 7. Randomly oriented nonwoven mats with fibers of uniform diameter were obtained with the binary system, as shown in Fig. 7(a, c and e). Moreover, there were numerous elliptical nanopores on the wrinkled fiber surface in Fig. 7(b, d and f). The average fiber diameter and the pore size are listed in Table 2. It is clearly noted that with the increase of the applied voltage, samples prepared from DCM/DMF show an increase in the average fiber diameter from 1.68 ± 0.17 to 2.18 ± 0.24 μm. The pore size increased when the applied voltage changed from 12 to 18 kV. However, no distinct difference was observed in the average diameter of the pore short axis compared to the average diameter of the pore long axis, which changed from 66 ± 18 nm to 141 ± 66 nm. The elliptical nanopores were attributed to the stretching of the electrical force; thus, the stronger stretch under high voltage compared to 12 kV leads to the larger size of the pores. Obviously, the high-magnification SEM of the fiber cross section demonstrated that the fibers were not collapsed during electrospinning, as seen in Fig. 7(h), resulting in cylindrically shaped fibers. Interestingly, a highly porous interior could be visibly seen at the cross section of a broken fiber (Fig. 7(g and h)). There was a good similarity in the PS/DMF/DCM system, which elucidated that the porous core was induced by VIPS.34
| Applied voltage (kV) | Average fiber diameter (μm) | Average diameter of pore long axis (nm) | Average diameter of pore short axis (nm) |
|---|---|---|---|
| 12 | 1.68 ± 0.17 | 66 ± 18 | 32 ± 5 |
| 15 | 1.96 ± 0.17 | 106 ± 44 | 30 ± 10 |
| 18 | 2.18 ± 0.24 | 141 ± 66 | 39 ± 10 |
3.2 Construction of primary structure
There are usually three typical primary structures obtained in electrospinning: beads, beads-on-string structure, and uniform fiber. In this work, the effects of the solvents on the morphologies of electrospun fibers are listed in Table 3. It is clear that beads-on-string and uniform fiber structures were observed with these solvents. Although the vapor pressure of the solvent determines the evaporation rate and drying time, the primary structures were not changed monotonously with the boiling point of the solvents. In addition, it is reported there are three reasons for the formation of beads during electrospinning. Firstly, insufficient chain overlap by low concentration or corresponding viscosity leads to the formation of polymer beads and fibers with beads by Rayleigh instability.6 Secondly, the bead-on-string structure prefers to form by the oscillation of Taylor cone and the instability of polymer jets induced by high voltage or solution conductivity.43 Finally, when the flow rate exceeds a critical value, the breaking of mass-balance resulted in sustained but unstable jets and beads-on-string structure is formed.44 It is insinuated that the surface tension, conductivity and viscosity of the solution affected by solvent are the key factors determining the primary fiber structure. The fiber morphology was not changed monotonously with solution conductivity and viscosity of different solvents, as shown in Table 3. It is reasonable to postulate that solution conductivity and viscosity have synergistic effects on the primary structure of electrospun fibers. Regarding PMMA/acetone system with high conductivity (7.02 × 10−5 S m−1) and relatively low viscosity (30.67 mPa s), the generation of beads was responsive to low viscosity without sufficient molecular chain entanglement. The viscoelastic force was insufficient to suppress the surface tension and shearing force, accordingly, beads were prone to be generated. However, the instability of the polymer jets induced by the high conductivity of the PMMA/DMF solution (1.52 × 10−4 S m−1) and the high surface tension of DMF was accounted for by the formation of beads on the fibers. The high viscosity of the PMMA/chloroform solution (210 mPa s) causes the polymer fluid jet to break up into small droplets, which were stretched into thin fibers by the electrical force. It could be the mechanism of the large fiber diameter distribution of PMMA fibers electrospun from chloroform.| System | Conductivity (S m−1) | Viscosity (mPa S) | Average fiber diameter (μm) | Fiber morphology |
|---|---|---|---|---|
| PMMA/DCM | 8.00 × 10−7 | 100.0 | 5.2–7.67 | Ribbon-like cross section, porous surface |
| PMMA/acetone | 7.02 × 10−5 | 30.67 | — | Beads-on-string structure, wrinkled surface |
| PMMA/chloroform | 2.60 × 10−6 | 210.0 | 2.94–3.75 | Ribbon-like cross section, porous surface |
| PMMA/THF | 2.63 × 10−4 | 54.00 | 1.73–2.08 | Round-shape cross section, wrinkled surface |
| PMMA/ethyl acetate | <1.00 × 10−7 | 65.00 | 6.00–7.00 | Ribbon-like cross section, porous surface |
| PMMA/DMF | 1.52 × 10−4 | 80.33 | — | Beads-on-string structure, wrinkled surface |
| PMMA/DCM/DMF | 6.55 × 10−5 | 96.67 | 1.68–2.18 | Round-shape cross section, porous surface and core |
Round-shaped fibers are the most commonly produced, with a few exceptions that fibers with collapsed ribbon-like cross-section were observed. Flat, ribbon-like fiber is a well-known phenomenon that has been observed in the manufacture of textile fibers by electrospinning, such as protein,45 carbon,46 PLLA,47 and PS.15,28 Ribbon-like PMMA fibers were routinely fabricated by electrospinning PMMA/DCM, PMMA/chloroform, and PMMA/ethyl acetate solutions in the present study. Li et al.48 developed a fiber-division model, resulting from coulombic repulsion and solvent vaporization to interpret the formation of ribbon-like Ce(NO3)3/PVP composite fibers, which elucidated positive charges redistributed on the sides of the jet due to perturbation or instability. In addition, coulombic repulsion made the fiber deform to have an elliptic or ribbon cross section. However, it is reasonable to postulate that in the study present study, “fiber-division model” is not the appropriate mechanism for ribbon-like fibers from electrospinning polymer solution with poor electrical conductivity. Koombhongse et al.5 proposed a mechanism for the formation of ribbon-shaped fibers whereby atmospheric pressure tended to collapse the skin as the solvent evaporated. Moreover, Lin and co-workers28,49 described that the flat, ribbon – shaped fiber resulted from atmospheric pressure obtained when the solvent evaporated more quickly than the rate at which air and vapor penetrated into the fiber. Pauchard et al.50 observed a similar phenomenon when a solvent evaporates from a sessile polymer solution drop, which was attributed to a well-known buckling instability. Pai et al.36,51 and Fashandi et al.34 claimed that the reason for ribbon-like fiber is due to buckling instability that occurs during the drying of the polymer jet. In order to describe the buckling instability, Pauchard et al.50 proposed two characteristic times, drying time (tD) and buckling time (tB), and Pai et al.36 developed another characteristic time, the time for phase separation (tPS). Both of these times are related to the evaporation rate, and tB is directly proportional to the mutual diffusion coefficient.50 Pai and his co-workers36,51,52 have theoretically analyzed that different cross-sections of fibers could be fabricated, accounting for the influences of drying and buckling. Regarding PMMA/DCM, PMMA/chloroform, and PMMA/ethyl acetate solutions, tPS < tB < tD, which indicates that the phase separation goes faster than both solvent drying and buckling instability, resulting in nanopores on the fiber surface. In addition, tB is smaller than tD, indicating that the buckling instability may be observed, resulting in collapsed ribbon-like fiber morphology.36,51 In addition, Pai et al.36 demonstrated that larger fibers tend to collapse due to longer drying time, which accounts for the fibers from these three systems with 3–8 μm average diameter to have collapsed ribbon-like structure. The tB of the other four systems, such as PMMA/THF, is comparable to their tD, demonstrating that the cylinder fiber should be obtained with wrinkled surfaces. Therefore, solvent volatility is important to the cross-sectional shape of the fibers.
3.3 Construction of hierarchical structure
In this work, it is regarded that the exact mechanism of hierarchical structure formation is complex, and TIPS may not be applicable to explain the formation mechanism of pores or wrinkles on fibers. This statement is in agreement with the general research on a variety of other polymer electrospun fibers, such as polyvinyl pyrrolidone (PVP), polyethylene oxide (PEO), and polyvinyl acetate (PVA), which revealed that neither interior pores nor porous surfaces were observed while volatile solvents were used in carrying out electrospinning in an air environment.27,53,54 The solid core sections, as shown in Fig. 1(c, f, i) and 5(b, d, f), prove that the pertinent explanation underlying the surface pores of PMMA fibers is not TIPS. It is reported that the isolated pores were also observed inside the fiber, when TIPS accounted for pore formation on PMMA,37 POM (Poly oxymethylene)14 and cellulose acetate (CA)31 fibers. Moreover, the “Breath Figure” model has been widely used to elucidate the mechanism for generation of pores on the fiber surface,55–57 which may not be applicable in this work. The reason is that gravity works as the driving force to maintain the water attached to the polymer surface. In other words, the pores resulting from the “Breath Figure” model appeared only on the upper side of the fiber, when the fiber flew horizontally to the target during electrospinning. Nevertheless, the pores emerged on the entire fiber surface. Even considering that the fiber ceaselessly rotated during flying to the collector over a short distance, it is impossible to form the pores on the entire fiber.21 On the other hand, water droplets were found to order in hexagonal array when condensed on a cold surface,58,59 which generally induced micro-sized pores. However, the pores created from phase separation were usually with a small diameter of nanoscale.14,60 The circular pores with size distributions in the range of 60–150 nm and elliptical nanopores with axis from tens of nanometers to 140 nm in the present study do not support that “Breath Figure” is the relevant mechanism for pore occurrence. Consequently, VIPS is responsible for the generation of nanopores on PMMA fibers with a relative humidity of 40% when a sufficient amount of water vapor diffuses into the solution. Porous PS,21,34 poly etherimide (PEI)61 fibers have been obtained by electrospinning at or above a relative humidity of 40%, whereas smooth fibers were fabricated at a low level of relative humidity, which were attributed to the penetration of a nonsolvent (water vapor in air) in the polymer solution and causing phase separation (VIPS).From the previous analysis, it is concluded that VIPS could be the most pertinent mechanism to explain the observation of porous or wrinkled fiber surfaces. To elucidate the formation of the featured fiber morphologies, a ternary phase diagram was shown in Fig. 8. There are three regions including a stable one phase region, metastable region and unstable region divided by the spinodal and binodal curve. When the jet ejects from the Taylor cone during electrospinning, the solvent evaporates from the polymer solution fluid involving flashing vaporization at the fiber surface and the diffusion from core to surface.28 Afterwards, water vapor, which is the nonsolvent of PMMA, diffuses into the vicinity of the jets. These processes bring the composition and temperature of the jet quite differently from the original solution, resulting in thermodynamic instability of the jet. The polymer solution passes through the binodal or spinodal curve of the phase diagram simultaneously, leading to phase separation that emerged in polymer-rich and polymer-lean domains. After the evaporation of solvent and water in combination with fiber stretching by electrical force, the polymer-rich phase forms a solid fiber matrix, whereas the solvent-rich phase evolves into pores with uniform distribution or wrinkles. When electrospun with DCM, chloroform, ethyl acetate, and binary solvent (DCM/DMF), the nucleation growth (NG) occurs as the solution passes through the binodal curve from stable region to metastable region, resulting in isolated circular pores on the fiber surface (path type II in Fig. 8). The PMMA/DMF, PMMA/THF, PMMA/acetone system acts as the path type I, indicating that the solution passes though the unstable region during electrospinning. The solution separated via spinodal decomposition (SD) in the unstable region results in the wrinkled fiber surface.34 No phase separation occurs if the solution composition is changing in the homogeneous region (path type III in Fig. 8), bringing about smooth fiber surface. In addition, some research groups have focused on the influence of relative humidity on fiber morphology, which proved the indispensable role of water vapor in producing porous polymer fibers.15,21,34 It has been reported that smooth and solid fibers were observed at low relative humidity (below 10%).36,56 In addition, it appears that the density of the surface pores is a response to the viscosity of the polymer-rich phase. Ethyl acetate is insoluble in water, which effectively hindered the water vapor from penetrating into the jet and inducing phase separation. Therefore, a fiber with few nanopores could be created by electrospinning PMMA/ethyl acetate solution. However, the high vapor pressure DCM, which is also insoluble in water, was used, and the evaporation of DCM absorbed a great amount of heat, cooled the nearby water vapor and began to condense in the vicinity of the jet interface.21 As a result, PMMA fibers with pores on the entire surface could be observed. In the binary solvent system, the high boiling DMF (426 K) and the low boiling DCM (313 K) diffuse from the core to the surface of the polymer jet with different rates, which induce jet instability and phase separation in the core. In addition, the precipitation of water vapor into the jet surface causes the phase separation. As a result, PMMA fibers with porous cores and surfaces were produced with DCM and DMF. This observation revealed some similarity to the investigation of electrospinning PS,28 CA,29 PLLA62 from binary solvent systems with different volatilities.
4. Conclusions
PMMA fibers were successfully produced by electrospinning through different solvents at 298 ± 2 K with a relative humidity of 38% ± 2%. In this study, uniform fiber mats were obtained from the PMMA/DCM, PMMA/chloroform, PMMA/THF, and PMMA/ethyl acetate system with a solution concentration of 20%, whereas the beads-on-string structure was observed by electrospinning with acetone and DMF. By analyzing the effect of solution conductivity and viscosity on fiber morphology, it was concluded that fiber morphology is not changed monotonously with varying solution conductivity and viscosity of different solvents. In addition, collapsed ribbon-like fibers were fabricated by electrospinning from DCM, chloroform, and ethyl acetate, which was attributed to the buckling instability. Flat ribbon-like fibers can be generated when drying time (tD) is greater than buckling time (tB).All of the electrospun PMMA fibers from these systems had hierarchical structures, which can be explained with the mechanism of vapor-induced phase separation (VIPS) under the high relative humidity of 38% ± 2%. Exposing the polymer solution to the water vapor (nonsolvent) atmosphere led to water penetration process-induced phase separation by the thermodynamic instability of polymer solution. Nanopores in round shapes with a distribution of 60–150 nm on the fiber surface were observed from PMMA/DCM and PMMA/ethyl acetate solutions. The morphology evolution could be explained by the nucleation growth (NG) mechanism during phase separation. Moreover, the increase of applied voltage does not exert significant influence on the fiber diameter, pore diameter, and pore amount. Similarly, with the NG mechanism and stretching of the electrical force, elliptical nanopores were observed on the fibers produced from PMMA/chloroform and PMMA/DMF/DCM solution. Furthermore, the wrinkled surface of PMMA fibers electrospun from acetone, THF, and DMF is the characteristic feature of spinodal decomposition (SD) mechanism.
Acknowledgements
The authors would like to acknowledge financial support from the following sources: the National Natural Science Foundation of China (no. 21376186), the Ministry of Education (Doctoral Special Research Foundation no. 20110201110032), China, and the Fundamental Research Funds for the Central Universities (New Teacher Research Support Plan no. 08141002, International Cooperation Project no. 2011jdhz37 and Integrated Cross Project xjj2014136 in Xi'an Jiaotong University), the Natural Science Basic Research Plan in Shaanxi Province of China (no. 2012JM2010), and the Ministry of Human Resources and Social Security of China (Science and Technology Project for Overseas Scholars, no. 19900001).References
- Z. M. Huang, Y. Z. Zhang, M. Kotaki and S. Ramakrishna, A review on polymer nanofibers by electrospinning and their applications in nanocomposites, Compos. Sci. Technol., 2003, 63, 2223–2253 CrossRef CAS
.
- H. Lou, W. Li, C. Li and X. Wang, Systematic investigation on parameters of solution blown micro/nanofibers using response surface methodology based on box-Behnken design, J. Appl. Polym. Sci., 2013, 130, 1383–1391 CrossRef CAS
.
- R. Nayak, R. Padhye, I. L. Kyratzis, Y. B. Truong and L. Arnold, Recent advances in nanofibre fabrication techniques, Text. Res. J., 2012, 82, 129–147 CrossRef CAS PubMed
.
- A. Formhals, Process and apparatus for preparing artificial threads, US Pat. 1975504, 1934
.
- S. Koombhongse, W. Liu and D. H. Reneker, Flat polymer ribbons and other shapes by electrospinning, J. Polym. Sci., Part B: Polym. Phys., 2001, 39, 2598–2606 CrossRef CAS
.
- P. Gupta, C. Elkins, T. E. Long and G. L. Wilkes, Electrospinning of linear homopolymers of poly(methyl methacrylate): exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent, Polymer, 2005, 46, 4799–4810 CrossRef CAS PubMed
.
- B. Ding, C. Li, Y. Miyauchi, O. Kuwaki and S. Shiratori, Formation of novel 2D polymer nanowebs via electrospinning, Nanotechnology, 2006, 17, 3685–3691 CrossRef CAS
.
- H.-S. Bae, A. Haider, K. M. K. Selim, D.-Y. Kang, E.-J. Kim and I.-K. Kang, Fabrication of highly porous PMMA electrospun fibers and their application in the removal of phenol and iodine, J. Polym. Res., 2013, 20, 158 CrossRef PubMed
.
- F. Iskandar, A. B. D. Nandiyanto, K. M. Yun, C. J. Hogan, K. Okuyama and P. Biswas, Enhanced photocatalytic performance of brookite TiO2 macroporous particles prepared by spray drying with colloidal templating, Adv. Mater., 2007, 19, 1408–1412 CrossRef CAS
.
- T. Zhao, Z. Liu, K. Nakata, S. Nishimoto, T. Murakami, Y. Zhao, L. Jiang and A. Fujishima, Multichannel TiO2 hollow fibers with enhanced photocatalytic activity, J. Mater. Chem., 2010, 20, 5095–5099 RSC
.
- H. Kokubo, B. Ding, T. Naka, H. Tsuchihira and S. Shiratori, Multi-core cable-like TiO2 nanofibrous membranes for dye-sensitized solar cells, Nanotechnology, 2007, 18, 165604 CrossRef
.
- M. F. Leong, K. S. Chian, P. S. Mhaisalkar, W. F. Ong and B. D. Ratner, Effect of electrospun poly (d,l-lactide) fibrous scaffold with nanoporous surface on attachment of porcine esophageal epithelial cells and protein adsorption, J. Biomed. Mater. Res., Part A, 2009, 89, 1040–1048 CrossRef PubMed
.
- M. Bognitzki, W. Czado, T. Frese, A. Schaper, M. Hellwig, M. Steinhart, A. Greiner and J. H. Wendorff, Nanostructured fibers via electrospinning, Adv. Mater., 2001, 13, 70–72 CrossRef CAS
.
- T. Kongkhlang, M. Kotaki, Y. Kousaka, T. Umemura, D. Nakaya and S. Chirachanchai, Electrospun polyoxymethylene: Spinning conditions and its consequent nanoporous nanofiber, Macromolecules, 2008, 41, 4746–4752 CrossRef CAS
.
- J. Zheng, H. Zhang, Z. Zhao and C. C. Han, Construction of hierarchical structures by electrospinning or electrospraying, Polymer, 2012, 53, 546–554 CrossRef CAS PubMed
.
- A. Gupta, C. D. Saquing, M. Afshari, A. E. Tonelli, S. A. Khan and R. Kotek, Porous nylon-6 fibers via a novel salt-induced electrospinning, Macromoleculars, 2009, 42, 709–715 CrossRef CAS
.
- Z. Wei, Q. Zhang, L. Wang, M. Peng, X. Wang, S. Long and J. Yang, Porous electrospun PES/PEG ultrafine fibers for the removal of endocrine disruptors, Sep. Sci. Technol., 2013, 48, 2287–2292 CrossRef CAS
.
- S. O. Han, W. K. Son, D. Cho, J. H. Youk and W. H. Park, Preparation of porous ultra-fine fibres via selective thermal degradation of electrospun polyetherimide/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) fibres, Polym. Degrad. Stab., 2004, 86, 257–262 CrossRef CAS PubMed
.
- L. Jun, Y. Zhang, Y. Hao, L. Cheng and J. J. Zhang, Preparation of porous electro-spun UPM fibers via photocrosslinking, J. Appl. Polym. Sci., 2009, 112, 2247–2254 CrossRef CAS
.
- W. S. Lyoo, J. H. Youk, S. W. Lee and W. H. Park, Preparation of porous ultra-fine poly (vinyl cinnamate) fibers, Mater. Lett., 2005, 59, 3558–3562 CrossRef CAS PubMed
.
- P. Lu and Y. Xia, Maneuvering the internal porosity and surface morphology of electrospun polystyrene yarns by controlling the solvent and relative humidity, Langmuir, 2013, 29, 7070–7078 CrossRef CAS PubMed
.
- Z. Shen, B. E. Thompson and M. A. McHugh, Electrospinning in near-critical CO2, Macromolecules, 2006, 39, 8553–8555 CrossRef CAS
.
- M. McHugh, Z. Shen, D. Gee, G. Karles, J. Nepomuceno and G. U. Huvard, Method of producing fibers by electrospinning at high pressures, US Pat. US7935298 B2, 2011
.
- S. Machmudah, H. Kanda, S. Okubayashi and M. Goto, Formation of PVP Hollow Fibers by Electrospinning in One-Step Process at Sub and Supercritical CO2, 2014 Search PubMed
.
- J. Liu, Z. Shen, S.-H. Lee, M. Marquez and M. A. McHugh, Electrospinning in compressed carbon dioxide: Hollow or open-cell fiber formation with a single nozzle configuration, J. Supercrit. Fluids, 2010, 53, 142–150 CrossRef CAS PubMed
.
- K. Nayani, H. Katepalli, C. S. Sharma, A. Sharma, S. Patil and R. Venkataraghavan, Electrospinning combined with nonsolvent-induced phase separation to fabricate highly porous and hollow submicrometer polymer fibers, Ind. Eng. Chem. Res., 2011, 51, 1761–1766 CrossRef
.
- S. Megelski, J. S. Stephens, D. B. Chase and J. F. Rabolt, Micro- and nanostructure surface morphology on electrospun polymer fibers, Macromolecules, 2002, 35, 8456–8466 CrossRef CAS
.
- J. Lin, B. Ding and J. Yu, Direct fabrication of highly nanoporous polystyrene fibers via electrospinning, ACS Appl. Mater. Interfaces, 2010, 2, 521–528 CAS
.
- A. Celebioglu and T. Uyar, Electrospun porous cellulose acetate fibers from volatile solvent mixture, Mater. Lett., 2011, 65, 2291–2294 CrossRef CAS PubMed
.
- X. Wu, L. Wang, H. Yu and Y. Huang, Effect of solvent on morphology of electrospinning ethyl cellulose fibers, J. Appl. Polym. Sci., 2005, 97, 1292–1297 CrossRef CAS
.
- S. O. Han, W. K. Son, J. H. Youk, T. S. Lee and W. H. Park, Ultrafine porous fibers electrospun from cellulose triacetate, Mater. Lett., 2005, 59, 2998–3001 CrossRef CAS PubMed
.
- D. Lubasova and L. Martinova, Controlled morphology of porous polyvinyl butyral nanofibers, J. Nanomater., 2011, 2011, 1–6 CrossRef PubMed
.
- X. Y. Ye, F. W. Lin, X. J. Huang, H. Q. Liang and Z. K. Xu, Polymer fibers with hierarchically porous structure: combination of high temperature electrospinning and thermally induced phase separation, RSC Adv., 2013, 3, 13851–13858 RSC
.
- H. Fashandi and M. Karimi, Pore formation in polystyrene fiber by superimposing temperature and relative humidity of electrospinning atmosphere, Polymer, 2012, 53, 5832–5849 CrossRef CAS PubMed
.
- C. J. Luo, M. Nangrejo and M. Edirisinghe, A novel method of selecting solvents for polymer electrospinning, Polymer, 2010, 51, 1654–1662 CrossRef CAS PubMed
.
- C.-L. Pai, M. C. Boyce and G. C. Rutledge, Morphology of porous and wrinkled fibers of polystyrene electrospun from dimethylformamide, Macromolecules, 2009, 42, 2102–2114 CrossRef CAS
.
- P. Dayal, J. Liu, S. Kumar and T. Kyu, Experimental and theoretical investigations of porous structure formation in electrospun fibers, Macromolecules, 2007, 40, 7689–7694 CrossRef CAS
.
- P. Dayal and T. Kyu, Porous fiber formation in polymer-solvent system undergoing solvent evaporation, J. Appl. Phys., 2006, 100, 043512–043516 CrossRef PubMed
.
- L. Li, R. Li, L. Liu and T. Fang, Producing PMMA fibers by electrospinning and model prediction, J. Chem. Ind. Eng., 2013, 64, 1869–1875 CAS
.
- I. Smallwood, Handbook of organic solvent properties, John Wley & Sons Inc., New York, 1996 Search PubMed
.
- S. Piperno, L. Lozzi, R. Rastelli, M. Passacantando and S. Santucci, PMMA nanofibers production by electrospinning, Appl. Surf. Sci., 2006, 252, 5583–5586 CrossRef CAS PubMed
.
- Y. F. Qian, Y. Su, X. Q. Li, H. S. Wang and C. L. He, Electrospinning of polymethyl methacrylate nanofibres in different solvents, Iran. Polym. J., 2010, 19, 123–129 CAS
.
- X. Zong, K. Kim, D. Fang, S. Ran, B. S. Hsiao and B. Chu, Structure and process relationship of electrospun bioabsorbable nanofiber membranes, Polymer, 2002, 43, 4403–4412 CrossRef CAS
.
- C. Zhang, X. Yuan, L. Wu, Y. Han and J. Sheng, Study on morphology of electrospun poly(vinyl alcohol) mats, Eur. Polym. J., 2005, 41, 423–432 CrossRef CAS PubMed
.
- L. Huang, R. A. McMillan, R. P. Apkarian, B. Pourdeyhimi, V. P. Conticello and E. L. Chaikof, Generation of synthetic elastin-mimetic small diameter fibers and fiber networks, Macromolecules, 2000, 33, 2989–2997 CrossRef CAS
.
- S. H. Park, C. Kim, Y. I. Jeong, D. Y. Lim, Y. E. Lee and K. S. Yang, Activation behaviors of isotropic pitch-based carbon fibers from electrospinning and meltspinning, Synth. Met., 2004, 146, 207–212 CrossRef CAS PubMed
.
- J. Zeng, X. Xu, X. Chen, Q. Liang, X. Bian, L. Yang and X. Jing, Biodegradable electrospun fibers for drug delivery, J. Controlled Release, 2003, 92, 227–231 CrossRef CAS
.
- M. M. Li, Y. Z. Long, H. X. Yin and Z. M. Zhang, Electrospun cerium nitrate/polymer composite fibres: synthesis, characterization and fibre-division model, Chin. Phys. B, 2011, 20, 048101–048108 CrossRef
.
- J. Y. Lin, Preparation of hierarchically nanostructured electrospun fibers and its application in oil/water separation, Doctoral Dissertation, Donghua University, 2012
.
- L. Pauchard and C. Allain, Buckling instability induced by polymer solution drying, Europhys. Lett., 2003, 62, 897–903 CrossRef CAS
.
- C.-L. Pai, Morphology and machanical properties of electrospun polymeric fibers and their nonwoven fabrics, PhD dissertation, Massachusetts Institute Of Technology, 2011
.
- L. Wang, C.-L. Pai, M. C. Boyce and G. C. Rutledge, Wrinkled surface topographies of electrospun polymer fibers, Appl. Phys. Lett., 2009, 94, 151916 CrossRef PubMed
.
- S. Vrieze, T. Camp, A. Nelvig, B. Hagström, P. Westbroek and K. Clerck, The effect of temperature and humidity on electrospinning, J. Mater. Sci., 2008, 44, 1357–1362 CrossRef PubMed
.
- E. S. Medeiros, L. H. C. Mattoso, R. D. Offeman, D. F. Wood and W. J. Orts, Effect of relative humidity on the morphology of electrospun polymer fibers, Can. J. Chem., 2008, 86, 590–599 CrossRef
.
- S. Honarbakhsh and B. Pourdeyhimi, Scaffolds for drug delivery, part I: electrospun porous poly(lactic acid) and poly(lactic acid)/poly(ethylene oxide) hybrid scaffolds, J. Mater. Sci., 2011, 46, 2874–2881 CrossRef CAS
.
- C. L. Casper, J. S. Stephens, N. G. Tassi, D. B. Chase and J. F. Rabolt, Controlling surface morphology of electrospun polystyrene fibers effect of humidity and molecular weight in the electrospinning process, Macromolecules, 2004, 37, 573–578 CrossRef CAS
.
- M. M. Demir, Investigation on glassy skin formation of porous polystyrene fibers electrospun from DMF, eXPRESS Polym. Lett., 2009, 4, 2–8 CrossRef
.
- K. Hiwatari, T. Serizawa, F. Seto, A. Kishida, Y. Muraoka and M. Akashi, Graft copolymers having hydrophobic backbone and hydrophilic branches XXXIV. Fabrication and control of honeycomb structure prepared from amphiphilic graft copolymers, Polym. J., 2001, 33, 669–675 CrossRef CAS
.
- D. Beysens and C. M. Knobler, Growth of breath figures, Phys. Rev. Lett., 1986, 57, 1433–1436 CrossRef
.
- M. Srinivasarao, D. Collings, A. Philips and S. Patel, Three-dimensionally ordered array of air bubbles in a polymer film, Science, 2001, 292, 79–83 CrossRef CAS PubMed
.
- H. Fashandi and M. Karimi, Comparative studies on the solvent quality and atmosphere humidity for electrospinning of nanoporous polyetherimide fibers, Ind. Eng. Chem. Res., 2014, 53, 235–245 CrossRef CAS
.
- S. Cao, B. Hu and H. Liu, Fabrication of nano-porous structured polylactide (PLLA) fibers through electrospinning, Acta Polym. Sin., 2010, 10, 1193–1198 CrossRef
.
| This journal is © The Royal Society of Chemistry 2014 |