Self-assembly of flower-like γ-AlOOH and γ-Al2O3 with hierarchical nanoarchitectures and enhanced adsorption performance towards methyl orange†
Jinchun Xiaoab,
Honghai Jib,
Zhiqi Shenb,
Weiya Yangb,
Changyou Guob,
Shaojun Wangb,
Xiwen Zhangb,
Rong Fuab and
Fengxiang Ling*b
aSchool of Chemistry and Material Science, Liaoning Shihua University, Fushun, 113001, China
bFushun Research Institute of Petroleum and Petrochemicals, SINOPEC, Fushun, 113001, China. E-mail: fengxiangling.fripp@gmail.com; Fax: +86 24 56429551; Tel: +86 24 56389578
First published on 29th July 2014
Abstract
A novel flower-like γ-AlOOH and γ-Al2O3 with hierarchical nanoarchitectures were successfully synthesized via the assembly of P123 and aluminium chloride with urea being used as a precipitating agent under hydrothermal conditions. The influence on morphology control was studied by altering the amount of P123 (Vp123) and molar ratio of CO(NH2)2/Al3+ (RUAl), implying that the increasing RUAl could lead to the crystal growth in the form of nanofilms or nanostrips and the addition of P123 could have a morphology-alteration impact on the 3D hierarchical architectures, and the possible formation mechanism was carefully studied. Further adsorption investigation indicates that the flower-like γ-Al2O3 exhibits an enhanced adsorption performance towards methyl orange as a result of the unique hierarchical structure, and the addition of P123 also has an drastic effect on the surface acidity of the as-prepared flower-like γ-Al2O3.
Introduction
In the last decades, metal oxides with hierarchical structures have drawn more attention for their nanometre-sized building blocks and overall micrometre-sized structure with the increase of varied applications ranging from adsorption, catalysis, energy conversion and storage to drug delivery, nanotechnology and biotechnology.1–4 Alumina and aluminium oxide hydroxides nanomaterials with heterogeneous hierarchical nanostructures have been investigated extensively for scientific and industrial applications5–13 due to their novel properties and promising applications. Especially, γ-AlOOH and γ-Al2O3 are used widely as catalysts, catalyst supports, adsorbents, ceramics, abrasives and filters.14–16Up to now, various hierarchical structured boehmite and γ-Al2O3, such as nanotubes,17 nanofibers,18 nanorods,19 nanowire,20 nanoplatelets and nanowires,21 nanostructured microspheres,22 hollow microspheres23–25 have been successfully achieved by various methods. Zhang et al.26 have synthesized a flower-like three-dimensional (3D) nanoarchitectures by using anhydrate AlCl3, during the synthetic process the ethanol–water solution were selected to slow down the strong hydrolyzation of the anhydrate AlCl3.
However, not only the novel properties and hierarchical structures have been paid attention to but also the environmental problems involving water treatment. Despite the above-mentioned successful demonstrations, it is still a challenge to obtain γ-AlOOH or γ-Al2O3 with unique morphology and enhanced physicochemical properties, which would possess unique properties in the applications of water contamination treatment. Herein, a novel flower-like γ-AlOOH and γ-Al2O3 3D nanoarchitectures were successfully synthesized by hydrothermal method, and the effect on the morphology of the molar ratio of CO(NH2)2/Al3+ (RUAl) and the amount of poly(ethylene glycol)-block-poly(propylene-glycol)-block-poly(ethylene glycol) (P123) were also carefully studied. On the basis of the control experiments and time-dependent experiments, we proposed a possible formation mechanism of the novel flower-like nanoarchitectures. Moreover, the as-prepared γ-Al2O3 was found to have an enhanced adsorption performance toward methyl orange with a rapid adsorption rate, indicating their promising applications in water contamination treatment.
Experiment section
Synthesis of flower-like 3D hierarchical nanoarchitectures
All chemicals were of analytical grade reagents and used as received without further purification. In a typical synthesis experiment, all experiments were performed under air atmosphere, 0.05 M aluminium chloride (AlCl3·6H2O) and 0.075 M urea (CO(NH2)2) was dissolved in mixed solvents of water (50 mL) under vigorous stirring for 5 min, then 5 mL alcohol solution of 0.1 g mL−1 P123 (alcohol was used to increase the solubility of P123) were added to, with vigorous stirring for another 5 min to ensure complete mixing. The solution were transferred into a Teflon-lined stainless autoclave. The autoclave was sealed and maintained at 150 °C for 24 h statically, and then was allowed to cool to room temperature naturally. Consequently, the white precipitated powder was obtained by filtration, washed by alcohol and distilled water several times. The sample was dried at 70 °C for 12 h, and then calcined at 600 °C in air for 4 h. Control experiments, as described above, but with the molar ratio of CO(NH2)2/Al3+ (RUAl) being adjusted to 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Sam. 1), 2.25
1 (Sam. 1), 2.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Sam. 2), 3
1 (Sam. 2), 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Sam. 3), 3.75
1 (Sam. 3), 3.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Sam. 4) at Vp123 = 5 and the amount of P123 (Vp123) being adjusted to 1 (Sam. 5), 3 (Sam. 6), 7 (Sam. 7), 0 mL (Sam. 8) at RUAl = 1.5, were also undertaken to study the specific effect of the urea and P123 on the morphology.
1 (Sam. 4) at Vp123 = 5 and the amount of P123 (Vp123) being adjusted to 1 (Sam. 5), 3 (Sam. 6), 7 (Sam. 7), 0 mL (Sam. 8) at RUAl = 1.5, were also undertaken to study the specific effect of the urea and P123 on the morphology.
Characterization
The crystal structure and phase purity of the product were examined by X-ray diffraction (XRD, D/max-2500, Cu-Kα radiation). An overview of the sample morphology was checked with a scanning electron microscope (SEM, JSM-7500F, JEOL) and transmission electron microscopy (TEM, JEM-2100, JEOL) analysis. Nitrogen adsorption and desorption isotherms were conducted at 77 K by using a Micrometrics ASAP-2420. Prior to the above measurements, the as-prepared samples had been degassed under vacuum at 300 °C for 400 min. Pyridine FTIR spectra were recorded on a Nicolet-6700 spectrometer. The samples were pressed into self-supporting discs, placed in an IR cell, and treated under vacuum (10−5 Torr) at 400 °C for 2 h. After cooling down to room temperature, the samples were exposed to pyridine vapor for 30 s. Then, IR spectra were recorded after evacuation (10−5 Torr) for 1 h at various temperatures.Absorption treatment of methyl orange
Treatment of adsorption performance was performed by adding 40 mg of as-prepared samples into 100 mL methyl orange (40 mg L−1) under vigorous stirring at room temperature. Analytical sample was taken from the suspension after desirable adsorption times and separated by filtration. The methyl orange concentration was monitored by a UV-vis spectrophotometer (UV-2550, Shimadzu, Japan). The characteristic adsorptions around 466 nm were chosen to monitor the adsorption process and the C/C0 was used to characterize the relative adsorption capacity (C0 and C represent the initial concentration and concentration after equilibrium adsorption treatment, respectively).Results and discussion
Structure property and morphology
Powder XRD was used to monitor the changes in the phase of the as-prepared and calcined samples. Fig. 1 shows the wide-angle XRD patterns of the Sam. 1 before (Fig. 1a) and after calcination (Fig. 1b). Fig. 1a shows all detectable peaks in this pattern, which can be assigned to orthorhombic γ-AlOOH (JCPDS card no. 21-1307), the results demonstrate that all γ-AlOOH nanostructures are well-crystallized and no evidence could be found for the existence of other impurities in the products. The broaden diffraction peaks indicate that the as-obtained samples were composed of small crystals with a crystalline structure in nanometer scale. We obtained the γ-AlOOH with the urea, which used as a Brőnsted base for coprecipitation of metal ions and synthesis of metal-oxides or hydroxide with tuning the pH value of the medium remains relatively low throughout the reaction process. The procedures of AlOOH preparation can be described as follows:27,28| NH2CONH2 + H2O → CO2 + 2NH3 | (1) |
| NH3 + H2O → NH4+ + OH− | (2) |
| Al3+ + 3OH− → Al(OH)3 | (3) |
| Al(OH)3 → AlOOH + H2O | (4) |
The virgin γ-Al2O3 (Fig. 1b) (JCPDS card no. 10-0425) were fabricated by calcination of the as-prepared γ-AlOOH at 600 °C for 4 h, in agreement with a previous report29 that the phase is yield via the dehydration process of AlOOH at temperatures between 400 °C and 700 °C.
Fig. 2 and S1† present SEM and TEM images of the 3D flower-like (Sam. 1) γ-Al2O3 and γ-AlOOH nanostructures, respectively. Fig. 2a and b shows that well-defined 3D hierarchical flower-like γ-Al2O3 are self-assembled into the nanostructures with an mean size about 1.5–2 μm can be clearly observed. Moreover, no other morphologies can be observed, indicating a well-ordered 3D hierarchical flower-like morphology of the as-prepared γ-Al2O3. The TEM images of the γ-Al2O3 flower-like nanostructures are shown in Fig. 2c and d, which suggesting that the entire structure of the architectures were composed of several bunches of flake-like nanopetals with the lengths around 1 μm and 100 nm in width, respectively. These nanopetals with sharp edges and corners made of several aggregated nanoparticles which may be extremely useful for the high BET surface areas and high value of pore volume (see below). In addition, these nanopetals connected to each other via the centre to fabricate 3D flower-like hierarchical structures.
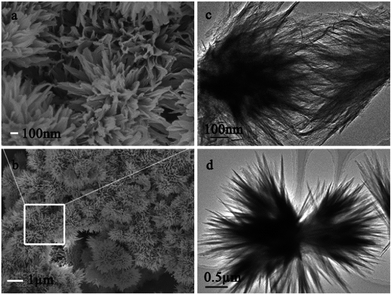 | ||
| Fig. 2 Typical SEM images (a and b) and TEM images (c and d) of the flower-like γ-Al2O3 (Sam. 1): (a) top-magnification image; (b) low-magnification image. | ||
It is significant to note that the 3D hierarchical flower-like nanostructures are considerable stable, even treated with the high temperature calcination, the integral morphology of the 3D hierarchical flower-like γ-AlOOH precursor nanostructures are not damaged after the calcination (as shown by Fig. 2a and S1 (ESI†)). The size and morphology of γ-Al2O3 are extreme similar to those of the γ-AlOOH, indicating that the size and morphology can be well preserved during the phase-transformation from γ-AlOOH to γ-Al2O3.
Surface area and porosity
Fig. 3 exhibits N2 adsorption–desorption isotherms and corresponding pore-size distribution (inset) of flower-like (Sam. 1) γ-Al2O3 (a) and γ-AlOOH (b), which exhibit similar Type IV shape isotherms with H1 hysteresis loops at relative pressures of P/P0 above 0.35 and 0.45, respectively. The samples show almost the same adsorption and desorption branch as well as the pore-size distribution, indicating that they possess the similar structure, which is in consistent with the SEM and TEM images (Fig. 2 and S1c†). Accordingly, the corresponding pore-size distribution were relatively narrow, and the samples all shown a narrow pore-size distribution centring at about 4 nm. It is may be the result of the coexistence of the high uniform nanopetals and flower-like nanostructures (Fig. 2), suggesting that the distribution of 4 nm originated from the nanopetals and flowers. The BET surface areas and the pore volumes of the flower-like γ-AlOOH and γ-Al2O3 are shown in Table S1.† The BET surface areas were as high as 186 m2 g−1, which is higher than that of the reported flower-like nanostructures.26 After calcination, the BET surface areas drastically increased from 186 to 260 m2 g−1 due to the phase transformation within the aluminium species, meanwhile, the pore volume increased from 0.31 to 0.49 cm3 g−1 and the average pore width decreased from 8.6 to 7.3 nm. | ||
| Fig. 3 N2 adsorption–desorption isotherms and corresponding BJH-pore size distribution (inset) of flower-like (Sam. 1) γ-Al2O3 (a) and γ-AlOOH (b). | ||
Surface acidity
It is well known that a alumina has considerable strong Lewis acidity (L acidity) and little Brőnsted acidity (B acidity) with the characteristic absorption peak at 1450 cm−1 and 1540 cm−1, respectively.30 Fig. 4 shows the FTIR spectra of samples after pyridine adsorption and evacuation at 160 °C, and the corresponding numbers of acid sites on the samples were shown in Table 1. The bands at 1450 and 1585 cm−1 are raised to the 8a and 19b modes of pyridine molecules interacting via hydrogen-bonding with the weakly acidic surface hydroxy-groups of γ-Al2O3 while the band at 1614 cm−1 are attributed to the same modes of pyridine molecularly coordinated with Al3+, acting as Lewis acid sites. The band at 1490 and 1540 cm−1 are assigned to the 19a and 19b modes of pyridinium cations, associated to a total proton transfer from the Brőnsted acidic surface OH− group to the basic molecule. The intensity of bands are in good agreement with the numbers of acid sites on the samples (Table 1). It is worth nothing that, the increasing amount of P123 have no regular relationship with the amount of total acid sites, while the Lewis acid sites reduced. Although the previous reports31 claimed that the total acid sites decreased severely after the calcination treatments, in this work, the acid sites can be preserved by the addition of P123. | ||
| Fig. 4 FTIR spectra of pyridine adsorption on the γ-Al2O3 samples after evacuation for 1 h at 160 °C. (a) Sam. 5; (b) Sam. 6; (c) Sam. 1; (d) Sam. 7. | ||
| Samples | Total acidity (mmol g−1) | L acidity (mmol g−1) | B acidity (mmol g−1) |
|---|---|---|---|
| Sam. 5 | 0.391 | 0.369 | 0.022 |
| Sam. 6 | 0.355 | 0.345 | 0.010 |
| Sam. 1 | 0.330 | 0.318 | 0.012 |
| Sam. 7 | 0.339 | 0.310 | 0.029 |
Effect on the morphology formation
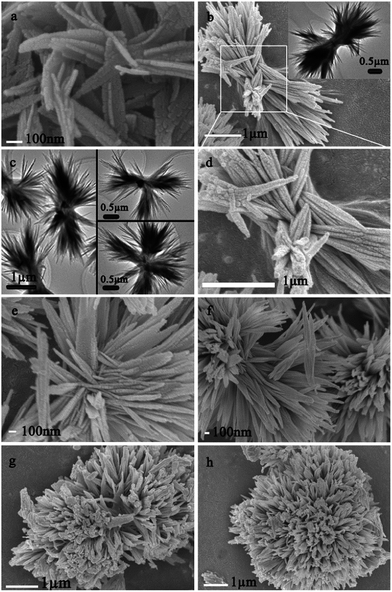
The molar ratio of CO(NH2)2/Al3+ (RUAl) and amount of P123 (Vp123) were found to be critical in the morphology control (shown in Fig. 5). First, Fig. 2 and 5a–c show the morphology of γ-Al2O3 obtained under the varied RUAl, while other synthesis parameters keep a constant. As shown in the Fig. 5a and b, a typical nanofilms obtained with RUAl = 2.25 (Sam. 2) and RUAl = 3 (Sam. 3), and Fig. 5c presents a nanostrip with the widths ranging from 20 to 40 nm. With a increasing RUAl, the morphology were changed from 3D hierarchical architectures (flower-like) to 1D architectures (nanostrip), which may raise by the PH value change of the solution. As show in the above eqn (1)–(4), the pH values must gradually increase as the increasing of the OH− content hydrolyzed by urea when the OH− content were excess to react with the Al3+. Generally, a slow hydrolysis of precursors may favour to the formation process of 1D oxide nanostructures such as nanowires and nanorods.32,33 Thus, we can draw a conclusion that the increasing RUAl hinder the hydrolysis of precursors, and result in the crystal growth in the form of nanofilms and nanostrips.Second, Fig. 2 and 5c–e show the morphology of the γ-Al2O3 obtained under the varied Vp123, while other synthesis parameters keep a constant. As shown in Fig. S2,† there were only irregular particles obtained without the addition of P123. However, with the increasing P123 content, novel 3D flower-like nanostructures were gradually obtained (Fig. 2 and 5e and f, Sam. 1 and Sam. 6 and 7) followed the faulty flower-like (Fig. 5d). When the Vp123 = 1, a faulty (Fig. 5d, Sam. 5) flower-like nanostructures were obtained. Fig. 5e shows the average diameter of flower-like nanostructures grew up to 2.5–3 μm as well as the lengths of the nanopetals grew up to 1–1.5 μm, which have the similar nanopetals as the novel flower-like. On further increase the Vp123 to 7.5, the increasing of the P123 amount increased the viscosity of the system, which would make the flowers swarm together by the connection of the nanopetals (Fig. 2e). Generally, the addition of P123 could make the morphology grew to 3D hierarchical architectures.
It can be found that the increasing RUAl could lead to the crystal growth in the formation of nanofilms or nanostrips, while the addition of P123 could have the morphology-alteration impact to 3D hierarchical architectures.
Formation mechanism
To further investigate the formation of flower-like nanostructure, the time-dependent experiments were also carried out by the SEM and TEM images (Fig. 6 and S3†). As shown in Fig. S3a,† amorphous aluminum hydroxide nanorods with diameters around 30 nm appears. Prolonged to 10 h, the nanorods gradually self-assembled into lager flake-like nanopetals (Fig. 6a) on the flowers (Fig. 2). As the evolution proceeded, spindle-like were self-assembled (Fig. 6b and d and inset) and, at the same time, some flowers were self-assembled by more nanopetals being self-assembled onto the spindles (Fig. 6c and inset). In the higher magnification SEM image (Fig. 6d), the self-assembled process can be clearly tell apart. Fig. 3d and 6b show the similar spindle-like nanostructures, indicating that when the reaction time prolonged to 12 h the dissolution procedure of P123 molecules in the system is the same as the system of Sam. 5. In addition, the experiments suggested that the dissolution of P123 molecules were a rather slow process. After 16 h, the faulty flower-like nanostructures (Fig. 6e and f) were self-assembled via the aggregation of numerous nanopetals. A further increasing in the reaction time up to 18 h, led to the formation of more uniform flower-like (Fig. 6g and h and S3b†).The structural evolution was a long process, which might be the result of the hydrolysis of urea and the dissolution of P123 molecules. From the morphologies of the samples at different evolutionary stages, a possible three-plot formation mechanism is proposed. (I) First, the irregular AlOOH was formed from the previously formed Al(OH)3 (eqn (1)–(3)) under the hydrothermal conditions. However, the amount of the AlOOH crystal at this time was small, and no precipitation was found, which is in well agreement with the time-dependent experiments of 6 h that we performed. As the OH− content increased by the hydrolysis of urea, the irregular AlOOH particles tended to spilt for the distorted hydrogen bonds and interaction between the solvent molecules and the surface OH− groups via hydrogen bonds, yields the 1D AlOOH nanorods (Fig. S3a†) with the rolling mechanism.34,35 (II) Then, the formed nanorods began to form nanopetals (Fig. 6a), possibly via an oriented-attachment process.36 (III) As the reaction proceeded, the P123 content was gradually increased in the reaction system and the number of P123 adsorbed on the surfaces of nanopetals was higher, which may result in self-assemble process of nanopetals into spindle-like structure (Fig. 6b–d and inset). It is believed that the flower-like ZnO (ref. 37) and CuS (ref. 38) structure are preferably formed from rods structure in order to minimize the interfacial free energy, which is also suitable for the formation of flower-like γ-Al2O3 structure. With a prolonged reaction time, the spindle-like finally self-assembled into novel flower-like (Fig. 2 and 6g and h) by van der Waals forces and the hydrogen bonding,36 which have the lower interfacial free energy. Our control experiments and time-dependent experiments agree well with the self-assembly formation mechanism of the flower-like nanostructure, suggesting the three-plot formation mechanism can be described as following Fig. 7.
Absorption application in water for methyl orange
The as-prepared flower-like γ-Al2O3 samples were used as adsorbents for potential application in water treatment. Owing to their unique hierarchical porous structure and textual properties.39–42 As show in Fig. 8B, it is clear that over 90.0% of the methyl orange had been adsorbed at room temperature after only 2–4 min, and an equilibrium established within 4 min. With the increasing of Vp123, the adsorption behaviors change slightly (Fig. 8B(b–d)) except for the Sam. 5. As show in Fig. 5d–f and 2, the faulty and uniform flower-like nanostructures may be a key effect on the adsorption behaviors, which exhibit the similar pore structure property (Table S1†). In contrast, the commercial γ-Al2O3 shown much lower adsorption efficiency (Fig. 8B(e)) than the flower-like nanostructures due to the novel morphology. However, the flower-like γ-AlOOH (Fig. 8B(b*)) exhibited an lowest adsorption efficiency, which had unique hierarchical structure, indicating that the γ-AlOOH have weaker adsorption properties than γ-Al2O3.41,42 The enhanced adsorption properties of γ-Al2O3 are mainly affected by the coordination of the unique hierarchical structure and chemical composition. | ||
| Fig. 8 UV-vis absorption spectra of methyl orange in the presence of the γ-Al2O3 (Sam. 1) after time intervals (A) and the adsorption rates (B) on different samples: (a) Sam. 5; (b) Sam. 1; (c) Sam. 6; (d) Sam. 7; (e) commercial γ-Al2O3; (b*) γ-AlOOH obtained before the calcination of the corresponding Sam. 1 (b). The inset in Fig. 8A and B show the partial enlarged spectra and an accurate measurement with a short time scale, respectively. | ||
Conclusion
A series of nanofilm, nanostrip, spindle-like and flower-like γ-AlOOH and γ-Al2O3 nanoarchitectures were successfully synthesized by a facile method. The influence in morphology control were studied by altering the amount of P123 (Vp123) and molar ratio of CO(NH2)2/Al3+ (RUAl), implying that the increasing RUAl could lead to the crystal growth in the formation of nanofilms or nanostrips and the addition of P123 could have the morphology-alteration impact to 3D hierarchical architectures. Moreover, the possible self-assembled formation mechanism were put forward by combining the control experiments and time-dependent experiments. The morphology of the flower-like nanoarchitectures was strongly dependent on the experimental conditions including the molar ratio of CO(NH2)2/Al3+, the amount of P123 and the reaction time. Further adsorption investigation indicates that the flower-like γ-Al2O3 have good adsorption properties involving with methyl orange. The novel flower-like γ-AlOOH and γ-Al2O3 are promising candidates for industrial application, such as adsorbents, catalyst, catalyst supports, ceramic, and optical nanodevices.Acknowledgements
This work was financially supported by the SINOPEC (no. 113027). The authors are grateful to Dr L. Wang for helpful discussions.Notes and references
- S. F. Xie, B. J. Zheng, Q. Kuang, X. Wang, Z. X. Xie and L. S. Zheng, CrystEngComm, 2012, 14, 7715 RSC.
- (a) J. S. Hu, L. S. Zhong, W. G. Song and L. J. Wan, Adv. Mater., 2008, 20, 2977 CrossRef CAS PubMed; (b) D. F. Zhang, H. Zhang, L. Guo, K. Zheng, X. D. Han and Z. Zhang, J. Mater. Chem., 2009, 19, 5220 RSC.
- P. Yu, X. Zhang, D. L. Wang, L. Wang and Y. W. Ma, Cryst. Growth Des., 2009, 9, 528 CAS.
- Y. Q. Wang, G. Z. Wang, H. Q. Wang, W. P. Cai, C. H. Liang and L. Zhang, Nanotechnology, 2009, 20, 155604 CrossRef PubMed.
- A. M. A. Cruz and J. G. Eon, Appl. Catal. A-Gen., 1990, 167, 203 CrossRef.
- D. Mishra, S. Anand, R. K. Panda and R. P. Das, Mater. Lett., 2000, 42, 38 CrossRef CAS.
- Y. Kim, C. Kim, I. Choi, S. Rengaraj and J. Yi, Environ. Sci. Technol., 2004, 38, 924 CrossRef CAS.
- K. V. P. M. Shafi, A. Ulman, J. Lai, N. L. Yang and M. H. Cui, J. Am. Chem. Soc., 2003, 125, 4010 CrossRef CAS PubMed.
- B. E. Yoldas, J. Mater. Sci., 1975, 10, 1856 CrossRef CAS.
- S. Music, O. Dragcevic and S. Popovic, Mater. Lett., 1999, 40, 269 CrossRef CAS.
- P. Raybaud, M. Digne, R. Iftimie, W. Wellens, P. Euzen and H. Toulhoat, J. Catal., 2001, 201, 236 CrossRef CAS.
- P. Kim, J. B. Joo, H. Kim, W. Kim, Y. Kim, I. K. Song and J. Yi, Catal. Lett., 2005, 104, 181 CrossRef CAS PubMed.
- S. M. Kim, Y. J. Lee, J. W. Bae, H. S. Potdar and K. W. Jun, Appl. Catal., A, 2008, 348, 113 CrossRef CAS PubMed.
- J. Dupont, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667 CrossRef CAS PubMed.
- Z. R. Zhang, R. W. Hicks, T. R. Pauly and T. J. Pinnavaia, J. Am. Chem. Soc., 2002, 124, 1592 CrossRef CAS PubMed.
- G. Paglia, E. S. Bozin and S. J. L. Billinge, Chem. Mater., 2006, 18, 3242 CrossRef CAS.
- D. Kuang, Y. P. Fang, H. Q. Liu, C. Frommen and D. Fenske, J. Mater. Chem., 2003, 13, 660 RSC.
- (a) D. Deng, R. Tang, X. Liao and B. Shi, Langmuir, 2008, 24, 368 CrossRef CAS PubMed; (b) W. Q. Cai, H. Q. Li and Y. Zhang, Colloids Surf., A, 2007, 295, 185 CrossRef CAS PubMed; (c) B. Gong, Q. Peng, J. S. Jur, C. K. Devine, K. Lee and G. N. Parsons, Chem. Mater., 2011, 23, 3476 Search PubMed.
- T. B. He, L. Xiang and S. L. Zhu, Langmuir, 2008, 24, 8284 CrossRef CAS PubMed.
- Z. Y. Tang, N. A. Kotov and M. Giersig, Science, 2002, 297, 237 CrossRef CAS PubMed.
- X. Y. Chen, H. S. Huh and S. W. Lee, Nanotechnology, 2007, 18, 285608 CrossRef.
- L. Zhang and Y. J. Zhu, J. Phys. Chem. C, 2008, 112, 16764 Search PubMed.
- X. Wang, P. Hu, F. L. Yuan and L. J. Yu, J. Phys. Chem. C, 2007, 111, 6706 CAS.
- S. Y. Gao, H. J. Zhang, X. M. Wang, R. P. Deng, D. H. Sun and G. L. Zheng, J. Phys. Chem. B, 2006, 110, 15847 CrossRef CAS PubMed.
- W. Q. Cai, J. G. Yu and S. Mann, Microporous Mesoporous Mater., 2009, 122, 42 CrossRef CAS PubMed.
- J. Zhang, S. J. Liu, J. Lin, H. S. Song, J. J. Luo, E. M. Elssfah, E. Ammar, Y. Huang, X. X. Ding, J. M. Gao, S. R. Qi and C. C. Tang, J. Phys. Chem. B, 2006, 110, 14249 CrossRef CAS PubMed.
- W. H. R. Shaw and J. J. Bordeaux, J. Am. Chem. Soc., 1955, 77, 4729 CrossRef CAS.
- S. Ramanathan, S. K. Roy, R. Bhat, D. D. Upadhyaya and A. R. Biswas, Ceram. Interfaces, 1997, 23, 45 CrossRef CAS.
- C. Misra, Industrial Alumina Chemicals, American Chemical Society, Washington DC, Ser. 184, 1986 Search PubMed.
- (a) E. P. Parry, J. Catal., 1963, 2, 371 Search PubMed; (b) G. Busca, Catal. Today, 1998, 41, 191 CrossRef CAS.
- X. R. Huang and X. H. Li, J. Mol. Catal., 2001, 15, 6 CAS.
- Y. Wan and D. Y. Zhao, Chem. Rev., 2007, 107, 2821 CrossRef CAS PubMed.
- Q. S. Huo, D. Y. Zhao, J. L. Feng, K. Weston, S. K. Buratto, G. D. Stucky, S. Schacht and F. Schuth, Adv. Mater., 1997, 9, 974 CrossRef CAS PubMed.
- A. C. Pierre and D. R. Uhlmann, J. Non-Cryst. Solids, 1986, 82, 271 CrossRef CAS.
- J. F. Banfield, S. A. Welch, H. Z. Zhang and T. T. Ebert, Science, 2000, 289, 751 CrossRef CAS.
- R. L. Penn and J. F. Banfield, Science, 1998, 281, 969 CrossRef CAS.
- R. X. Shi, P. Yang, J. R. Wang, A. Y. Zhang, Y. N. Zhu, Y. Q. Cao and Q. Ma, CrystEngComm, 2012, 14, 5996 RSC.
- H. Qi, J. F. Huang, L. Y. Cao and J. P. Wu, Key Eng. Mater., 2012, 512, 265 CrossRef.
- J. B. Lian, X. C. Duan, J. M. Ma, P. Peng, T. I. Kim and W. J. Zheng, ACS Nano, 2009, 3, 3749 CrossRef CAS PubMed.
- J. B. Fei, Y. Cui, X. H. Yan, W. Qi, Y. Yang, K. W. Wang, Q. He and J. B. Li, Adv. Mater., 2008, 20, 452 CrossRef PubMed.
- Y. Wang, W. Li, X. L. Jiao and D. R. Chen, J. Mater. Chem. A, 2013, 1, 10720 CAS.
- W. Q. Cai, Y. Z. Hu, J. Chen, G. X. Zhang and T. Xia, CrystEngComm, 2012, 14, 972 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05343e |
| This journal is © The Royal Society of Chemistry 2014 |