Open Access Article
Open Access ArticleA new route to nanoscale ceramics in asymmetric reaction fields of carbon nanospaces†
Tomonori
Ohba
*,
Yuki
Ohyama
and
Hirofumi
Kanoh
Graduate School of Science, Chiba University, 1-33 Yayoi, Inage, Chiba 263-8522, Japan. E-mail: ohba@pchem2.s.chiba-u.ac.jp
First published on 21st July 2014
Abstract
We fabricated nanoscale BaTiO3 and its asymmetric crystal structure was obtained using an asymmetric reaction field in carbon nanospaces. The nano-crystal phases changed from symmetric to asymmetric crystals with decreasing crystallite size. The fabrication of an asymmetric crystal in a nanospace can be adapted for various ceramic fabrications.
The fabrication of nanoscale materials (nanomaterials) has attracted attention in fundamental and applied research, because nanomaterials have very different physical properties from those of single crystals and coarse-grained polycrystals.1,2 The interfaces of nanomaterials are primarily responsible for their novel properties, based on high reactivities and large surface areas.3–6 Control of these interfaces or the grain size is therefore necessary for the preparation of widely applicable nanomaterials.7,8 Barium titanate (BaTiO3), a perovskite-type material, is one of the most common electronic ceramic materials because it can be used in electro-optic devices, capacitors, and transducers. Nanoscale BaTiO3 is expected to have novel structural and physical properties: grain-size dependence of structural transformations and lowering of the Curie temperature with decreasing grain size.9–12 The design of nanoscale BaTiO3 has contributed to significant developments in ferroelectrics and the miniaturization of electronic devices. Much attention has focused on the preparation of nanoscale BaTiO3 by hydrothermal, solvothermal, sol–gel, and chemical precipitation methods instead of BaTiO3 crystal preparation by high-temperature solid-state reactions.13–17 Sol–gel and sol–gel solvothermal reactions have the advantage of uniform nanoscale crystal productions.10,13,18,19 Here, we describe size-dependent nanocrystalline phases of BaTiO3 and BaTiO3 with an anomalous structure, prepared under asymmetric reaction fields in the nanospaces of activated carbon fibers (ACFs), which are known to have abundant carbon nanospaces, with strong molecular fields for adsorption, separation, storage, and chemical reactions.20–22 A sol–gel solvothermal method was adopted for preparation of nanoscale BaTiO3 confined in nanospaces of ACFs, because the subnanometer scale precursor by a sol–gel method could enter into those nanospaces.17
Crystalline-BaTiO3 was prepared using a solid-state reaction by heating at 1473 K (see the Supplementary Information for details). The powder XRD pattern of crystalline-BaTiO3 (Fig. 1a) showed a highly crystalline BaTiO3 structure without any impurities, and matched previously reported patterns.23,24 The crystallite size of crystalline-BaTiO3 was 1270 nm, decided from the SEM images. Solid-state reactions at 1073 and 1173 K also produced BaTiO3 crystals (micro-BaTiO3) without impurities, but the peaks in the XRD patterns were wider than those for crystals prepared at 1473 K, as shown in Fig. 1b. The crystallite sizes for micro-BaTiO3 preparation at 1073 and 1173 K were 94 and 110 nm, respectively, evaluated from the XRD peaks using the Scherrer equation.25,26 The crystallite sizes of micro-BaTiO3 were apparently smaller than that of crystalline-BaTiO3. Thus, crystallite size could be controlled by the heating temperature in solid-state reactions. Fig. 1c shows XRD patterns of BaTiO3 crystals (nano-BaTiO3) prepared by a sol–gel method at 400 K using a Ti concentration of 50–400 mM. The crystal sizes of nano-BaTiO3 were approximately 5 nm. The XRD pattern peaks of confined-BaTiO3 were further broadened, as shown in Fig. 1d, because these BaTiO3 crystals are nanoscale. The XRD patterns also included peaks from ACFs. The XRD pattern of ACF had broad peaks corresponding to the 002 and 10 layers of graphitic materials, with crystallite sizes of 1.8 and 4.1 nm, respectively, determined using the Scherrer equation. Thus, the in-plane size of graphitic unit was 4.1 nm, as shown in Fig. S2c.† The average crystallite sizes of crystalline, micro-, nano-, and confined-BaTiO3 are summarized in Fig. 1e. BaTiO3 confined in nanospaces gave crystals smaller than 3 nm under all preparation conditions. Specifically, the crystallite sizes were nearly 1 nm when the Ti concentrations were weaker than 50 mM. This is a result of confined effect in nanospaces. The unit sizes of these nanospaces were approximately 4.1 × 4.1 × 1.3 nm3 (a schematic image of confined-BaTiO3 is shown in Fig. S2†). The BaTiO3 crystals were therefore smaller than 3.6 nm when the crystals were prepared in nanospaces, despite the same preparation method. In other words, the crystal sizes of confined-BaTiO3 were restricted to less than 3.6 nm, although the preparation method was the same as for nano-BaTiO3, with crystallite sizes of 5 nm. The crystal sizes of nanoscale ceramics can therefore be controlled by choosing nanospaces of appropriate sizes.
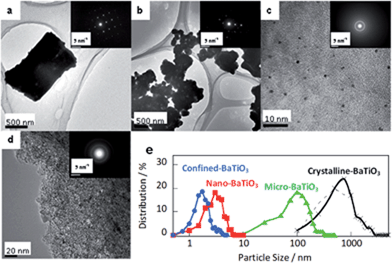
The BaTiO3 crystals were observed using TEM and SEM, as shown in Fig. 2 and S3.† BaTiO3 crystals prepared by a solid-state reaction at 1173 K, a sol–gel solvothermal reaction using a Ti concentration of 50 mM, and a sol–gel solvothermal reaction in nanospaces using a Ti concentration of 50 mM were used as crystalline-, micro-, nano-, and confined-BaTiO3, respectively. Crystalline- and micro-BaTiO3 were cuboid or polyhedral, but nano- and confined-BaTiO3 were roughly spherical. Electron diffraction showed spot patterns for crystalline-BaTiO3, spot and ring patterns for micro-BaTiO3, and only ring patterns for nano- and confined-BaTiO3. These patterns are roughly typical of tetragonal-phase BaTiO3. Energy-dispersive spectroscopy mapping showed that these particles consist of Ba, Ti, and O (Fig. S3†). For confined-BaTiO3, Ba, Ti, and O were also found in the ACFs. The particle size distributions in Fig. 2e were calculated from more than 200 particles of each type of BaTiO3 crystal in TEM images, assuming spherical particles. The particle sizes were approximately 800 ± 520, 110 ± 65, 3.3 ± 1.3, and 1.8 ± 0.6 nm for crystalline-, micro-, nano-, and confined-BaTiO3, respectively, calculated on the assumption of a Gaussian distribution; the average sizes were 1060, 137, 3.6, and 2.1 nm, respectively. The obtained particle sizes correspond well to the crystallite sizes from XRD analysis in Fig. 1e. The particle sizes were also evaluated from the Brunauer–Emmett–Teller surface areas obtained from N2 adsorption isotherms, and assuming spherical particles, as shown in Fig. S4.† The average particle sizes were 750, 150, 3.1, and 3.6 nm for crystalline-, micro-, nano-, and confined-BaTiO3, respectively. The particle sizes evaluated from TEM, SEM, and N2 adsorption isotherms agree well with the crystallite sizes obtained using XRD for the corresponding crystals, as shown in Fig. S4d† (details are shown in Fig. S5†). The crystal sizes obtained using XRD were therefore supported by particle size evaluation using TEM, SEM and N2 adsorption isotherms.
The peak positions of the XRD profiles were slightly shifted to lower angles when the crystal size decreased (Fig. 1 and S5†). The lower angle shifts of the peak positions indicated that the corresponding lattice spaces were expanded. The relationships between crystallite size and lattice space are shown in Fig. 3a. The (222) and (220) layers rarely changed in crystalline- and micro-BaTiO3 (crystallite size less than 90 nm), whereas the (200) layer of micro-BaTiO3 expanded by 0.4% to give a cubic form, as discussed later. All the lattice spaces of nano-BaTiO3 (crystallite size between 5 and 30 nm) expanded by nearly 1%, and the (222) and (220) layers expanded significantly in confined-BaTiO3 (crystallite size less than 3 nm), although the (200) layer rarely changed to that of nano-BaTiO3. These results suggest that BaTiO3 nanocrystals such as nano- and confined-BaTiO3 form anomalous phases or crystal structures. The crystal structures of representative BaTiO3 crystals, which were the same as those used in the TEM observations, were determined by fitting simulated XRD patterns to the experimental ones (Fig. S6†). Crystalline-, micro-, nano-, and confined-BaTiO3 have the tetragonal phase with the P4/mmm space group, cubic phase with the Pm3m space group, tetragonal phase with the P4/mmm space group, and trigonal phase with the R3m space group, respectively. The peak widths were corrected using the Scherrer equation based on the experimental crystallite sizes. The peak positions for all BaTiO3 crystals coincided with each other in experimental and simulated XRD patterns. The crystalline-BaTiO3 (size 1000 nm) had a tetragonal phase (a = b = 0.3995 nm and c = 0.4037 nm), corresponding to reported values.27,28 The crystal structure of micro-BaTiO3 (100 nm in size) was transformed to the cubic phase (a = b = c = 0.401 nm). Structural transformations from tetragonal to cubic phases with decreasing crystallite size have also been reported elsewhere.29 However, nano-BaTiO3 (size 5–30 nm) had a tetragonal phase, like crystalline-BaTiO3, but larger crystal parameters (a = b = 0.40 nm and c = 0.42 nm). The confined-BaTiO3 (size 1–3 nm) was assigned a trigonal phase (a = b = c = 0.41 nm and γ = 88°). The trigonal phase is usually observed below 200 K for BaTiO3 crystals.30–32 A novel trigonal structure was therefore observed at room temperature for nanoscale BaTiO3. The appearance of the trigonal structure could be the result of two effects: nanosized crystal formation and an asymmetric reaction field in carbon nanospaces.
The BaTiO3 crystal structures were also examined using Raman spectroscopy (Fig. 4).29,31,33 Crystalline-BaTiO3 has the typical BaTiO3 peaks in the tetragonal phase: ν1(LO) = 720 cm−1, ν1(TO) = 520 cm−1, ν2(LO, TO) = 307 cm−1, and ν4(LO) = 267 cm−1. The peaks for the ν1(TO) and ν4(LO) modes are unchanged for micro-BaTiO3, whereas those for the ν1(LO) and ν2(LO, TO) modes decrease significantly, suggesting a cubic phase in micro-BaTiO3. Some Raman activities of the peaks for the ν1(LO) and ν2(LO, TO) modes decrease as a result of Ti disorder in the nominal cubic phase. For nano-BaTiO3, only the sharp peak for the ν3(TO) mode is observed. Confined-BaTiO3 has no obvious peaks in the Raman spectrum. However, after removal of ACFs by combustion at 673 K for 48 h, a small sharp peak for the ν3(TO) mode and broad peaks for the ν4(LO), ν1(TO), and ν1(LO) modes9 appear. These peaks are assigned to the trigonal phase of the BaTiO3 crystal and verify the presence of BaTiO3 crystals in the carbon nanopores. The BaTiO3 crystals of nano- and confined-BaTiO3 had unique Raman spectral features; these peaks could not be simply assigned to bulk crystal phases, but the trigonal phase was expected for confined-BaTiO3.
Conclusions
Our studies demonstrate the preparation of extremely small BaTiO3 crystals, with a crystallite size of approximately 1 nm. The crystal phase exhibits structural diversity even at room temperature, from tetragonal to cubic, tetragonal, and trigonal phases, with decreasing crystal size, associated with anomalous expansion of lattice spaces. The appearance of an anomalous trigonal phase of BaTiO3 confined in carbon nanospaces is the result of nanocrystallization in an asymmetric reaction field in these nanospaces and anomalous stabilization of the BaTiO3 nanocrystals, because the trigonal phase is only observed below 200 K in typical BaTiO3 crystals. Carbon nanospaces provide a strong asymmetric reaction field; therefore, asymmetric structures appear as a result of symmetry collapse, leading to a cryogenic phase in this case. These findings are the first demonstration of the preparation of extremely small perovskite-type nanoceramics and structural diversity with decreasing crystallite size, and anomalous lattice expansion.9,33–35 This method for nanocrystal preparation and nanostructuration could be used to synthesize various ceramics. Further studies on the piezoelectric properties and photorefractive effects are necessary to assess the performance of nanoscale asymmetric BaTiO3.TEM observations were performed at the Chemical Analysis Center, Chiba University. This research was supported by a JSPS KAKENHI Grant Number 26706001 and Research Fellowships from the Futaba Electronics Memorial Foundation, Foundation for the JGC-S Scholarship Foundation, Promotion of Ion Engineering, and Murata Science Foundation.
Notes and references
- B. S. Guiton and P. K. Davies, Nat. Mater., 2007, 6, 586–591 CrossRef CAS PubMed.
- P. M. Rorvik, T. Grande and M. A. Einarsrud, Adv. Mater., 2011, 23, 4007–4034 CrossRef CAS PubMed.
- H. Goesmann and C. Feldmann, Angew. Chem., Int. Ed., 2010, 49, 1362–1395 CrossRef CAS PubMed.
- E. Roduner, Chem. Soc. Rev., 2006, 35, 583–592 RSC.
- Y. Wang, J. Zhang and Y. Zhao, Nano Lett., 2007, 7, 3196–3199 CrossRef CAS PubMed.
- I. Szlufarska, A. Nakano and P. Vashishta, Science, 2005, 309, 911–914 CrossRef CAS PubMed.
- Z. Shen, Z. Zhao, H. Peng and M. Nygren, Nature, 2002, 417, 266–269 CrossRef CAS PubMed.
- I.-W. Chen and X.-H. Wang, Nature, 2000, 404, 168–171 CrossRef CAS PubMed.
- Z. Zhao, V. Buscaglia, M. Viviani, M. T. Buscaglia, L. Mitoseriu, A. Testino, M. Nygren, M. Johnsson and P. Nanni, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 024107 CrossRef.
- M. H. Frey and D. A. Payne, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 3158–3168 CrossRef CAS.
- Y.-I. Kim, J. K. Jung and K.-S. Ryu, Mater. Res. Bull., 2004, 39, 1045–1053 CrossRef CAS PubMed.
- S.-G. Kwon, K. Choi and B.-I. Kim, Mater. Lett., 2006, 60, 979–982 CrossRef CAS PubMed.
- S. O'. Brien, L. Brus and C. B. Murray, J. Am. Chem. Soc., 2001, 123, 12085–12086 CrossRef.
- T. M. Stawski, S. A. Veldhuis, O. F. Göbel, J. E. Ten Elshof and D. H. A. Blank, J. Am. Ceram. Soc., 2010, 93, 3443–3448 CrossRef CAS PubMed.
- Y. Mao, S. Mao, Z.-G. Ye, Z. Xie and L. Zheng, Mater. Chem. Phys., 2010, 124, 1232–1238 CrossRef CAS PubMed.
- X. Zhu, J. Wang, Z. Zhang, J. Zhu, S. Zhou, Z. Liu and N. Ming, J. Am. Ceram. Soc., 2008, 91, 2683–2689 CrossRef CAS PubMed.
- T. M. Stawski, S. A. Veldhuis, R. Besselink, H. L. Castricum, G. Portale, D. H. A. Blank and J. E. ten Elshof, J. Phys. Chem. C, 2011, 115, 20449–20459 CAS.
- Y. V. Kolen'ko, K. A. Kovnir, I. S. Neira, T. Taniguchi, T. Ishigaki, T. Watanabe, N. Sakamoto and M. Yoshimura, J. Phys. Chem. C, 2007, 111, 7306–7318 Search PubMed.
- M. Veith, S. Mathur, N. Lecerf, V. Huch, T. Decker, H. P. Beck, W. Eiser and R. Haberkorn, J. Sol-Gel Sci. Technol., 2000, 17, 145–158 CrossRef CAS.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- S. Sircar, T. Golden and M. Rao, Carbon, 1996, 34, 1–12 CrossRef CAS.
- G. Che, B. B. Lakshmi, E. R. Fisher and C. R. Martin, Nature, 1998, 393, 346–349 CrossRef CAS PubMed.
- Y. Zhang, L. Wang and D. Xue, Power Technol., 2012, 217, 629–633 CrossRef CAS PubMed.
- D. Liu, Y. Yan and H. Zhou, J. Am. Ceram. Soc., 2007, 90, 1323–1326 CrossRef CAS PubMed.
- A. Patterson, Phys. Rev., 1939, 56, 978 CrossRef CAS.
- H. Borchert, E. V. Shevchenko, A. Robert, I. Mekis, A. Kornowski, G. Grübel and H. Weller, Langmuir, 2005, 21, 1931–1936 CrossRef CAS PubMed.
- J. Harada, T. Pedersen and Z. Barnea, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1970, 26, 336–344 CrossRef CAS.
- W.-S. Cho, J. Phys. Chem. Solids, 1998, 59, 659–666 CrossRef CAS.
- M. B. Smith, K. Page, T. Siegrist, P. L. Redmond, E. C. Walter, R. Seshadri, L. E. Brus and M. L. Steigerwald, J. Am. Chem. Soc., 2008, 130, 6955–6963 CrossRef CAS PubMed.
- G. Kwei, A. Lawson, S. Billinge and S. Cheong, J. Phys. Chem., 1993, 97, 2368–2377 CrossRef CAS.
- C. Perry and D. Hall, Phys. Rev. Lett., 1965, 15, 700–702 CrossRef CAS.
- P. W. Forsbergh Jr, Phys. Rev., 1949, 76, 1187 CrossRef.
- V. Buscaglia, M. T. Buscaglia, M. Viviani, L. Mitoseriu, P. Nanni, V. Trefiletti, P. Piaggio, I. Gregora, T. Ostapchuk, J. Pokorný and J. Petzelt, J. Eur. Ceram. Soc., 2006, 26, 2889–2898 CrossRef CAS PubMed.
- S. Lin, T. Lü, C. Jin and X. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 134115 CrossRef.
- X. Wang, X. Deng, H. Wen and L. Li, Appl. Phys. Lett., 2006, 89, 162902–162903 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Schematic image of preparation of BaTiO3, characterization of confined-BaTiO3, microscopic images of BaTiO3, N2 adsorption isotherms of BaTiO3 samples at 77 K, those XRD patterns, simulated XRD patterns, and Raman Spectroscopy. See DOI: 10.1039/c4ra05311g |
| This journal is © The Royal Society of Chemistry 2014 |