Nanoengineered CdSe quantum dot–montmorillonite composites: an efficient photocatalyst under visible light irradiation†
Abstract
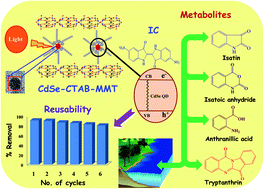
In this paper, we have developed an efficient electrostatic self-assembly strategy for the synthesis of a CdSe–CTAB–MMT composite that contains well-dispersed CdSe quantum dots (CdSe QDs) within the MMT structure. These nanocomposites are characterised by XRD, TEM, Raman, DRS and photoluminescence studies which indicate that 3–5 nm sized CdSe QDs are intercalated within lamellar sheets of MMT. Visible light induced photocatalytic activities of the composites exhibit decolourisation of Indigo Carmine (IC) solution (100 mg L−1) within 30 min. at 1.0 g L−1 catalyst loading. This feature is attributed to separation of photogenerated electron–hole pairs, enhanced interlayer spacing (7.2 A°), higher specific surface area and better adsorption capacity of the MMT. The involvement of reactive oxygen species (ROS) in the photodegradation process is ascertained by addition of selective quenchers such as NaN3 (for singlet oxygen), benzoquinone (for O2˙−), ascorbic acid (for OH˙) and KI (for h+). It is observed that singlet oxygen and photogenerated h+ do not contribute towards degradation; rather O2˙− is a prominent species that degrades ∼74% of IC while the remaining part is oxidized by OH˙. The photodegradation pathway involves desulphonation of IC followed by its oxidation to isatin, anthranilic acid, tryptanthrin and isatoic anhydride. The antibacterial studies of degraded IC solution as well as chemoinformatics studies suggest that these metabolites are non-toxic in nature. These catalysts remain active for up to 6 cycles with a marginal decrease in their removal capacity that can be ascribed to inhibition of photocorrosion even after successive exposure to light. Thus, nano-engineered CdSe-composites may be regarded as efficient photocatalysts that have potential applications in sustainable development towards continuous removal of organic dyes from aqueous streams.

 Please wait while we load your content...
Please wait while we load your content...