Ionization energies and photoelectron spectra of fat-soluble vitamins in the gas phase: a theoretical study†
Fatemeh Abyar and
Hossein Farrokhpour*
Chemistry Department, Isfahan University of Technology, Isfahan, 84156-83111, Iran. E-mail: farrokhphossein@gmail.com; h-farrokh@cc.iut.ac.ir; Fax: +98 311 3912350; Tel: +98 311 3913243
First published on 25th July 2014
Abstract
The electronic structures and photoelectron spectra of several fat-soluble vitamins including A (all-trans-retinol and its two derivatives, 13-cis-retinoic acid and all-trans-retinoic acid), D2, D3, E (consisting of α-tocopherol, β-tocopherol, γ-tocopherol and δ-tocopherol) and K were studied theoretically in this work. The vertical ionization energies of these compounds, including considerations of their electron correlations were calculated in the gas phase. The direct symmetry-adapted cluster/configuration interaction method, which employs single and double excitation operators (direct-SAC-CI SD-R), and the D95(df,pd) basis set were used for the calculations. The results indicate that more than one conformer contributes to the photoelectron spectrum of vitamin A, all-trans-retinoic acid, vitamin D2 and D3, suggesting that there is more than one biologically active form for each of these vitamins. The photoelectron spectrum of each vitamin was simulated and assigned, and the previously reported assignments from the literature were revisited. The ionization of vitamin D from highest occupied molecular orbit − 2 (HOMO − 2) and the lone electron pairs of oxygen was found to not take place below 10 eV. The first ionization band of vitamin E was assigned to the ionization from πC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and π*C
C and π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of its aromatic ring, unlike the previous assignment relating this ionization band to the lone electron pair of oxygen. In addition, the ionizations of vitamin A and its derivatives from the lone electron pairs of oxygen were found to not occur below 11 eV in the gas phase.
C of its aromatic ring, unlike the previous assignment relating this ionization band to the lone electron pair of oxygen. In addition, the ionizations of vitamin A and its derivatives from the lone electron pairs of oxygen were found to not occur below 11 eV in the gas phase.
Introduction
Vitamins are important micronutrients needed by the body in small amounts for different activities throughout the human body.1 They are divided into two groups: water-soluble, which include the B-complex and C vitamins, and fat-soluble vitamins including A, D, E and K.2 Vitamin A has a key role in different biological processes related to growth, cellular differentiation and interactions of cells with each other or with the extracelluar matrix.3 D vitamins are classified as steroid hormones because their molecular structures and biological activities are similar to steroids.4 The most well known of the D vitamins regulate the activity of Ca2+ and PO43− in the gut and kidneys.5 Vitamin D3 is converted to 1, 25-dihydroxycholecalciferol (1,25-(OH)2D3), which represents an active form of the vitamin and is a recognized nuclear receptor.5 Vitamin K is an essential cofactor in the production of proteins that bind calcium, supporting cardiovascular and bone health.6 Vitamin K2 is more efficient than K1 because it remains in the body longer and is active in more tissues.7 Vitamin E benefits the body by acting as an antioxidant and protecting vitamins A and C, red blood cells and essential fatty acids from destruction.8 Vitamin E serves as an antioxidant, protecting the body from damage from free radicals, and is also important for protecting cell membranes.9 Vitamins are extremely sensitive to light; water-soluble light sensitive vitamins include C, B12, B6, B2 and B9,10 and fat-soluble light sensitive vitamins include A, K and E.9 Most studies on the interaction of light with vitamins investigate the wavelength range from 290–700 nm, which includes both ultraviolet (UV) and visible light.9 For example, Kagan et al.11 investigated the effect of UV light on vitamin E, finding that this vitamin may act in two conflicting ways upon solar illumination in skin. In addition to its antioxidant function as a peroxyl radical scavenger, it may act as an endogenous photosensitizer, enhancing light-induced oxidative damage.10The intrinsic properties of biomolecules, which are hidden in the complex media of natural biological systems, can be revealed by theoretical and experimental studies conducted in the gas phase, in an isolated environment.12 In this case, a detailed understanding of the structures and dynamics of biomolecules together with the distinction between the intrinsic properties and those due to environmental interactions can be obtained. For example, the effects of bulk hydration and the interaction of other ions in the biological environment cause significant changes in the electronic structures of biological molecules. This in turn affects the chemical and physical properties of the molecules. Changes in the electronic structure of molecules in biological media are accompanied by changes in their orbital energies and possible reordering of the orbital energies. This has an effect on the vertical ionization energies and vertical excitation energies of the molecules. Comparing the valence ionization and excitation spectrum of a biomolecule in the gas phase with those in solution provides an understanding of how the biomolecule bonds and interacts with its surroundings. For example, Ghosh et al. theoretically studied the effect of hydration on the vertical ionization energy of thymine.13 They found that microsolvation reduces the ionization energy by about 0.1 eV per water molecule, while the first solvation shell increases the ionization energy by 0.1 eV compared to the ionization energy in the gas phase. As an another example, Slavicek et al. used both theoretical and experimental techniques to study the effect of solvation with water on the vertical ionization energies of cytidine and deoxythymidine in comparison to the gas phase.14 They reported that upon bulk hydration, the ionization potential of the base becomes insensitive to the presence of the sugar and phosphate, unlike that which is observed in the gas phase. Based on these studies, the ionization energies of biomolecules in the gas phase are very important.
The gas phase ionization energy is one of the most important and fundamental properties of vitamins, and these energies could be used to better understand some of the biological phenomena contributed by these molecules. For example, the vertical ionization energies of different forms of vitamin E can be used as diagnostic indicators for the variation in activation energy associated with the antioxidant reaction of this vitamin.15 In addition, measuring the ionization energies of vitamins provides information about the oxidative potential of these molecules and can be used as a scale for comparing their oxidation potential in electrochemical reactions. The assignment of the photoelectron spectra of vitamins is also important for elucidating the mechanism of vitamin damage caused by photoionization because it determines where ionization occurs in the vitamin molecule. Knowing the location of ionization in the different ionic states of a vitamin allows us to obtain information about how the vitamin is fragmented in its excited ionic states after ionization.
One of the best methods for measuring the ionization energies of molecules in the gas phase is photoelectron spectroscopy (PES).16–18 The most challenging part of gas phase PES, especially for large biomolecules, is the decomposition and degradation of the sample before it reaches the gas phase. In this case, calculating the gas phase ionization energies and photoelectron spectra of biomolecules with a very accurate computational method is useful and provides information about gas phase ionization. Although there have been many experimental studies on the interaction of UV light with vitamins in solution, there are a limited number of studies in the literature related to the ionization of vitamins in the gas phase using UV light and X-ray radiation. Jericevic et al.19 recorded the He–I photoelectron spectra for some vitamin A derivatives including trans-retinoic acid, trans-retinal and β-carotene in the gas phase. Katsumata and Ikehata recorded the first He–I photoelectron spectrum of vitamin A in the gas phase.20 Novak and Potts studied the electronic structures of vitamins D2 and D3 using a PES technique5 and found that, based on their similar photoelectron spectra, D vitamins have steroid structures. There are no high level theoretical calculations on the ionization energies and photoelectron spectra of vitamin D and its derivatives in the literature. Nagaoka et al.15 recorded the He–I photoelectron spectra of vitamin E and its derivatives, but there is no experimental photoelectron spectrum for vitamin K in the literature. The aim of this current work is: (i) to calculate the vertical ionization energies of fat-soluble vitamins including A (all-trans-retinol and its two derivatives, 13-cis-retinoic acid and all-trans-retinoic acid), D2, D3, E (consisting of α-tocopherol, β-tocopherol, γ-tocopherol and δ-tocopherol) and K, taking into consideration their electron correlations to study their electronic structures; and (ii) to simulate and assign their photoelectron spectra.
Computational methods
The direct symmetry-adapted-cluster – configuration interaction – single-double-R (SAC-CI-SD-R) method considering the single and double excitation operators was employed in this work. The direct SAC-CI method is a version of SAC-CI-SD-R that uses a direct configuration interaction like algorithm combined with a perturbation selection technique that is especially efficient for large molecules.21–27 One advantage of this method is that the computational time is shorter compared to that of the standard version of the SAC-CI code. It should be mentioned that the direct version of SAC-CI predicts the relative energies of ionization bands very well, which has been confirmed by the calculation of ionization energies and photoelectron spectra for caffeine, xanthine and hypoxanthine in our previous study.28 An active space consisting of the same number of unoccupied orbitals was used in all calculations; only the 1s orbital was frozen as a core orbital. The calculated SAC wave function was chosen as the wave function of the ground electronic state for the SAC-CI calculations. The D95(df,pd) basis set was used in all calculations, and the absence of diffuse functions in this basis set was compensated for by the addition of more polarization functions. The geometries of the conformers of each vitamin were optimized at the B3LYP/6-31+G(d) level of theory, and their standard Gibbs free energies were calculated at the same level of theory to obtain their Boltzmman population ratios (BPR) in the gas phase. The calculated BPR of the conformers were used to simulate their photoelectron spectra. The ionization cross sections were calculated using the monopole approximation,29 which allows the correct estimation of the relative intensities of ionization bands. Furthermore, natural bonding orbital (NBO) calculations using Gaussian NBO (version 5)30 were performed at the Hartree–Fock (HF) level of theory using the D95(df,pd) basis set to determine the localization of the canonical molecular orbitals at the ground electronic state for spectral band assignment. All of the calculations were performed using the Gaussian Quantum Chemistry Package.31Results and discussion
Vitamin D
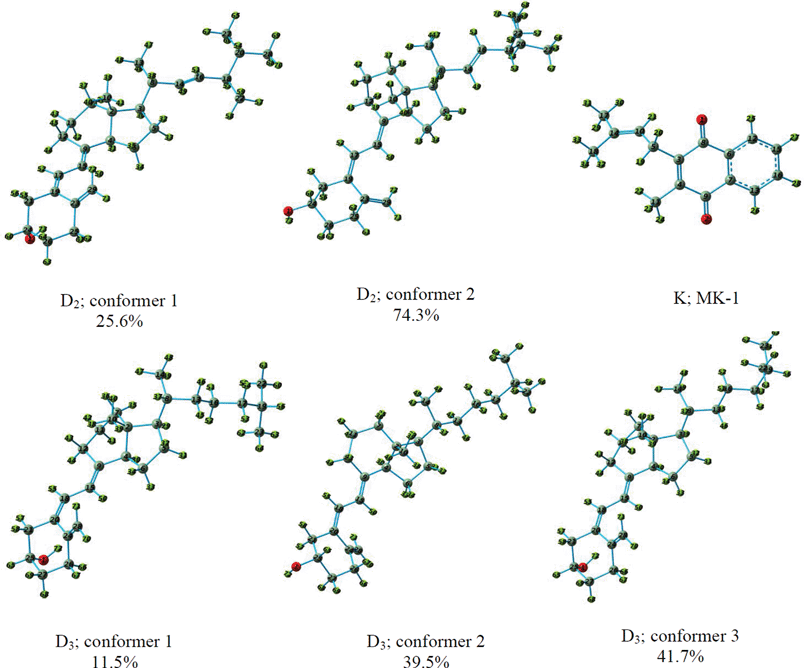
The results of the calculations for vitamin D2 and D3 are presented in this section. Generally, there are twenty and eight conformers for vitamin D2 and D3, respectively. The thermochemistry calculations performed in this work showed that only two and three conformers are populated in the gas phase for vitamin D2 and D3 at 406 K, respectively (Fig. 1). These conformers are also responsible for the biological activity of vitamins D2 and D3 in solution. Fig. 1 shows that there are three distinct moieties within the molecular structure of vitamin D: the rings, the side chain and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds.5 The population ratios of the conformers have also been reported in Fig. 1. The selected temperature for the thermochemistry calculations was equal to that selected by Novak and Potts5 for the sublimation of D vitamins in order to record their photoelectron spectra. Table S1† reports the calculated HF energies of the molecular orbitals of the conformers of vitamins D2 and D3 obtained from the HF calculations using the D95(df,pd) basis set. In addition, the results of NBO calculations to obtain the localizations of molecular orbitals involved in the ionization have been included in this table. Fig. S1† demonstrates the shape of the molecular orbitals of vitamins D2 and D3. Fig. 2 and 3 show the calculated photoelectron spectra of the conformers of vitamins D2 and D3, respectively; they also show their Boltzmann weighted photoelectron (BWP) spectra and compare them with the experimental photoelectron spectra.5
C bonds.5 The population ratios of the conformers have also been reported in Fig. 1. The selected temperature for the thermochemistry calculations was equal to that selected by Novak and Potts5 for the sublimation of D vitamins in order to record their photoelectron spectra. Table S1† reports the calculated HF energies of the molecular orbitals of the conformers of vitamins D2 and D3 obtained from the HF calculations using the D95(df,pd) basis set. In addition, the results of NBO calculations to obtain the localizations of molecular orbitals involved in the ionization have been included in this table. Fig. S1† demonstrates the shape of the molecular orbitals of vitamins D2 and D3. Fig. 2 and 3 show the calculated photoelectron spectra of the conformers of vitamins D2 and D3, respectively; they also show their Boltzmann weighted photoelectron (BWP) spectra and compare them with the experimental photoelectron spectra.5
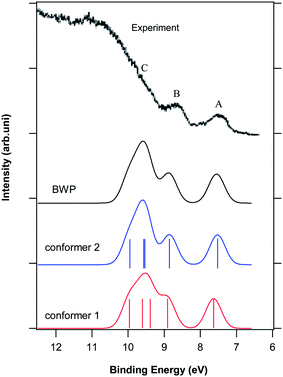 | ||
| Fig. 2 Comparison of the calculated photoelectron spectra and the BWP spectrum of the vitamin D2 conformers with the experimental photoelectron spectrum of this vitamin.5 Vertical lines show the calculated positions and intensities of ionization bands. The letters A, B and C indicate the dominant features in the experimental spectrum. | ||
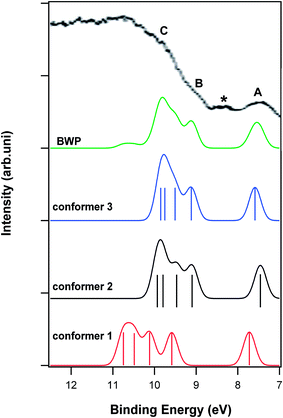 | ||
| Fig. 3 Comparison of the calculated photoelectron spectra and the BWP spectrum of the vitamin D3 conformers with the experimental photoelectron spectrum of this vitamin.5 Vertical lines show the calculated positions and intensities of ionization bands. The letters A, B and C indicate the dominant features in the experimental spectrum. | ||
Novak and Potts reported the first separate He–I photoelectron spectra of vitamins D2 and D3.5 They also performed semi-empirical Austin Model 1 (AM1) calculations to obtain the ionization and molecular orbital energies of vitamins D2 and D3. It should be mentioned that they did not perform conformational analysis on these vitamins to determine their ionization energies and conformer populations in the gas phase. Therefore, the ionization energies of these vitamins were calculated considering only one geometrical structure for each vitamin in the work of Novak and Potts. In addition, the molecular structures used for calculating the ionization energies in their work were optimized using molecular mechanics, and the observed features in the recorded photoelectron spectra were assigned based on Koopmans' approximation. Considering the above deficiencies, we decided in this work to calculate and assign the photoelectron spectra of vitamins D2 and D3 using a theoretical method that considers the electron correlations.
Fig. 2 indicates five ionization bands for the two conformers of vitamin D2 below 10 eV, while Novak and Potts predicted four ionization bands in this region based on Koopmans' approximation. In addition, the observed relative energies and intensities of the calculated ionization bands of conformers 1 and 2 are different. To obtain the calculated photoelectron spectrum of vitamin D2, the sum of the Boltzmann weighted individual calculated spectra of conformers 1 and 2 was considered (indicated by BWP in Fig. 2). As can be seen, there is an excellent agreement between the BWP spectrum and the experimental spectrum of vitamin D2 in Fig. 2. It is important to note that the experimental photoelectron spectrum of vitamin D2 is very similar to the calculated spectrum of conformer 2. This observation is in agreement with the population ratios of the vitamin D2 conformers obtained in this work (25.6% conformer 1; 74.3% conformer 2). Therefore, the assignment of the experimental photoelectron spectrum of D2 is explained based on the SAC-CI and NBO results for conformer 2.
Feature A of the experimental spectrum is related mainly to the first ionization band of conformers 1 and 2. The wave function of the first ionic state of D2 is a single HF-ionized determinant related to highest occupied molecular orbital (HOMO), which is a π molecular orbital due to π(C9–C15), π(C17–C19) and π(C23–C29) of the triene system (Tables S1† and 1). The calculated first ionization energy of conformer 2 obtained using the direct SAC-CI and HF methods are 5.945 and 7.75 eV, respectively. The position of feature A in the experimental spectrum of vitamin D2 is approximately 7.528 eV, while the first ionization energy of vitamin D2 calculated by Novak and Potts is approximately 8.72 eV. Thus, considering the electron correlations for vitamin D2 decreases the ionization energy. Therefore, the BWP spectrum of vitamin D2 (Fig. 2) was shifted by 1.58 eV towards the higher binding energy to match the position of the first peak of the BWP spectrum with the position of feature A in the experimental spectrum. The difference between the calculated and experimental first ionization energy of vitamin D2 can be attributed primarily to the intrinsic error present in the direct SAC-CI SD-R method. The average error of the direct SAC-CI method is approximately 0.5 eV in small molecules because of the cut-off approximation in the unlinked terms; this error increases with the size of the molecule.32 However, comparison of the BWP spectrum with the experimental spectrum shows that the direct SAC-CI SD-R method with the D95(df,pd) basis set can predict the relative energies and intensities of the photoelectron lines quite accurately. The numbers in parenthesis in Table 1 indicate the calculated ionization energies of the vitamin D conformers considering the value of the energy shift.
| State (2A) | Main electronic configuration | Ionization energy (eV) | Intensity | ||
|---|---|---|---|---|---|
| Vitamin D2 | Conformer 2 | 1 | 0.94(HOMO) | 5.945 (7.528) | 0.914 |
| 2 | −0.89(HOMO − 1) + 0.24(HOMO − 3) | 7.274 (8.854) | 0.913 | ||
| 3 | −0.9(HOMO − 4) + 0.21(HOMO − 3) | 7.957 (9.537) | 0.904 | ||
| 4 | −0.20(HOMO − 2) − 0.69(HOMO − 3)−0.51(HOMO − 4) − 0.30(HOMO − 1) | 7.990 (9.57) | 0.904 | ||
| 5 | −0.49(HOMO − 3) + 0.72(HOMO − 4) | 8.373 (9.953) | 0.900 | ||
| Vitamin D3 | Conformer 2 | 1 | 0.94(HOMO) | 5.858 (7.458) | 0.914 |
| 2 | 0.92(HOMO − 1) | 7.498 (9.098) | 0.902 | ||
| 3 | −0.87(HOMO − 2) + 0.30(HOMO − 3) | 7.868 (9.468) | 0.903 | ||
| 4 | 0.53(HOMO − 4) − 0.64(HOMO − 3) | 8.196 (9.796) | 8.196 | ||
| 5 | 0.52(HOMO − 3) + 0.72(HOMO − 4) | 8.332 (9.932) | 0.898 | ||
| Conformer 3 | 1 | 0.94(HOMO) | 5.987 (7.587) | 0.914 | |
| 2 | 0.39(HOMO − 1) − 0.84(HOMO − 2) | 7.518 (9.118) | 0.902 | ||
| 3 | 0.38(HOMO − 2) + 0.84(HOMO − 1) | 7.906 (9.506) | 0.905 | ||
| 4 | 0.21(HOMO − 2) + 0.70(HOMO − 3) − 0.23(HOMO − 4) − 0.48(HOMO − 5) | 8.153 (9.753) | 0.901 | ||
| 5 | −0.88(HOMO − 3) − 0.21(HOMO − 4) | 8.250 (9.85) | 0.903 | ||
| Vitamin K (MK-1) | 1 | −0.67(HOMO) + 0.57(HOMO − 4) + 0.24(HOMO − 3) | 7.386 | 0.899 | |
| 2 | −0.87(HOMO − 1)-0.26(HOMO − 3) −0.24(HOMO − 2) | 7.819 | 0.907 | ||
| 3 | 0.75(HOMO − 2) + 0.47(HOMO − 3) − 0.25(HOMO − 4) | 8.142 | 0.909 | ||
| 4 | 0.67(HOMO − 3) + 0.41(HOMO − 2) + 0.35(HOMO − 1) + 0.33(HOMO − 5) | 8.432 | 0.897 | ||
| 5 | 0.67(HOMO − 4) − 0.65(HOMO) | 8.472 | 0.891 | ||
The second ionization band of conformers 1 and 2 corresponds to feature B of the experimental spectrum. The second ionization band of vitamin D2 should occur from HOMO − 1 based on Koopmans' approximation, however, the SAC-CI result shows that this ionization can also originate from HOMO − 3 as well as HOMO − 1 because of the presence of electron correlations. HOMO − 1 is a π molecular orbital primarily localized on the C14![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C16 bond of the side chain of the molecule. The assignment of the first and second ionization bands of vitamin D2 are in agreement with the assignment performed by Novak and Potts.5 Notably, the third ionization band of vitamin D2 originates mostly from HOMO − 4, unlike the prediction by Koopmans' approximation (Table 1). HOMO − 4 is a π molecular orbital related only to π(C9–C15) and π(C17–C19) of the triene (Table S1†). This assignment is different to that reported by Novak and Potts.5 The wave function of the fourth ionization band is a linear combination of four single HF-ionized determinants including HOMO − 1, HOMO − 2, HOMO − 3 and HOMO − 4, demonstrating that the amount of electron correlations have been increased in this ionic state (see Table 1). One of the most important points related to vitamin D2 is that its ionization from HOMO − 2 and the lone electron pairs of its OH group do not occur below 10 eV.
C16 bond of the side chain of the molecule. The assignment of the first and second ionization bands of vitamin D2 are in agreement with the assignment performed by Novak and Potts.5 Notably, the third ionization band of vitamin D2 originates mostly from HOMO − 4, unlike the prediction by Koopmans' approximation (Table 1). HOMO − 4 is a π molecular orbital related only to π(C9–C15) and π(C17–C19) of the triene (Table S1†). This assignment is different to that reported by Novak and Potts.5 The wave function of the fourth ionization band is a linear combination of four single HF-ionized determinants including HOMO − 1, HOMO − 2, HOMO − 3 and HOMO − 4, demonstrating that the amount of electron correlations have been increased in this ionic state (see Table 1). One of the most important points related to vitamin D2 is that its ionization from HOMO − 2 and the lone electron pairs of its OH group do not occur below 10 eV.
Three conformers of vitamin D3 are observed in the gas phase (Fig. 1). The difference between vitamin D2 and D3 is mainly related to the side chain (there is no C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the side chain of D3). Fig. 3 shows the calculated photoelectron spectra of the vitamin D3 conformers together with their BWP spectrum. The experimental spectrum of vitamin D3 recorded by Novak and Potts5 is also included in this figure. The calculated spectra needed to be shifted by 1.6 eV towards the higher binding energy in order to match the experimental spectrum. The experimental photoelectron spectrum of D3 is similar to that of D2, however, the first peak in the spectrum of D3 is broader than that in the spectrum of D2.5 This difference has been attributed to the more varied gas phase conformer population for vitamin D3 compared to D2,5 which is in agreement with the thermochemistry results obtained in this work, suggesting that the number of gas phase populated conformers of D3 is higher than for D2. Comparison of theoretical results with experimental results shows that feature A of the vitamin D3 experimental spectrum is composed only of the first ionization band of the conformers. Fig. 3 shows that the ionization energies of conformer 1 are higher than those of the other two conformers. For example, the order of the first ionization energies of the vitamin D3 conformers are: conformer 1 > conformer 3 > conformer 2. The difference between the first ionization energies of the vitamin D3 conformers causes the broadening of feature A in the spectrum of vitamin D3 compared to that in the spectrum of vitamin D2.
C bond in the side chain of D3). Fig. 3 shows the calculated photoelectron spectra of the vitamin D3 conformers together with their BWP spectrum. The experimental spectrum of vitamin D3 recorded by Novak and Potts5 is also included in this figure. The calculated spectra needed to be shifted by 1.6 eV towards the higher binding energy in order to match the experimental spectrum. The experimental photoelectron spectrum of D3 is similar to that of D2, however, the first peak in the spectrum of D3 is broader than that in the spectrum of D2.5 This difference has been attributed to the more varied gas phase conformer population for vitamin D3 compared to D2,5 which is in agreement with the thermochemistry results obtained in this work, suggesting that the number of gas phase populated conformers of D3 is higher than for D2. Comparison of theoretical results with experimental results shows that feature A of the vitamin D3 experimental spectrum is composed only of the first ionization band of the conformers. Fig. 3 shows that the ionization energies of conformer 1 are higher than those of the other two conformers. For example, the order of the first ionization energies of the vitamin D3 conformers are: conformer 1 > conformer 3 > conformer 2. The difference between the first ionization energies of the vitamin D3 conformers causes the broadening of feature A in the spectrum of vitamin D3 compared to that in the spectrum of vitamin D2.
In a similar way to vitamin D2, the first ionization band of vitamin D3 is related to the ionization from HOMO, which is a π molecular orbital related to the triene system of the molecule (Tables 1 and S1†). Table S1† shows that the HOMOs of vitamin D3 conformers 2 and 3 are mostly localized on π(C15![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C9) and π(C18
C9) and π(C18![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C20). A small feature observed in the experimental spectrum of vitamin D3 (assigned with an asterisk in Fig. 3) is at first glance assumed to be the second ionization band of vitamin D3, however, the calculated BWP spectrum presented in this work does not show or confirm this feature. Feature B of the experimental spectrum is only related to the second ionization band of conformers 2 and 3. Therefore, it is possible to follow the change in the total population of conformers 2 and 3 relative to conformer 1 with temperature using the change in intensity of this feature detected by photoelectron spectroscopy. The wave function of the second ionization band of conformer 2 is mainly related to HOMO − 1; in contrast, it is related to both HOMO − 1 and HOMO − 2 (most contribution) for conformer 3. It can be seen that Koopmans' approximation is not valid for conformer 3, whereas it is for conformer 2. The HOMO − 1s of conformers 2 and 1 are mostly composed of π(C24
C20). A small feature observed in the experimental spectrum of vitamin D3 (assigned with an asterisk in Fig. 3) is at first glance assumed to be the second ionization band of vitamin D3, however, the calculated BWP spectrum presented in this work does not show or confirm this feature. Feature B of the experimental spectrum is only related to the second ionization band of conformers 2 and 3. Therefore, it is possible to follow the change in the total population of conformers 2 and 3 relative to conformer 1 with temperature using the change in intensity of this feature detected by photoelectron spectroscopy. The wave function of the second ionization band of conformer 2 is mainly related to HOMO − 1; in contrast, it is related to both HOMO − 1 and HOMO − 2 (most contribution) for conformer 3. It can be seen that Koopmans' approximation is not valid for conformer 3, whereas it is for conformer 2. The HOMO − 1s of conformers 2 and 1 are mostly composed of π(C24![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C28) and π(C9
C28) and π(C9![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C15), respectively, and HOMO − 2 has σ character. The third ionization band of conformer 2 is related to HOMO − 2, whereas it is related to HOMO − 1 in conformer 3, however, there is a little contribution from HOMO − 3 and HOMO − 2 to the main configuration of this ionic state for conformers 2 and 3, respectively. The wave function of the fourth ionization band of conformer 2 is a linear combination of HOMO − 4 and HOMO − 3, while in conformer 3 it is related to HOMO − 3 and HOMO − 5 with nearly the same contribution. HOMO − 3 is a π molecular orbital related to C18
C15), respectively, and HOMO − 2 has σ character. The third ionization band of conformer 2 is related to HOMO − 2, whereas it is related to HOMO − 1 in conformer 3, however, there is a little contribution from HOMO − 3 and HOMO − 2 to the main configuration of this ionic state for conformers 2 and 3, respectively. The wave function of the fourth ionization band of conformer 2 is a linear combination of HOMO − 4 and HOMO − 3, while in conformer 3 it is related to HOMO − 3 and HOMO − 5 with nearly the same contribution. HOMO − 3 is a π molecular orbital related to C18![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C20 and C9
C20 and C9![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C15. The shapes of the molecular orbitals of the two vitamin D3 conformers are illustrated in Fig. S1.†
C15. The shapes of the molecular orbitals of the two vitamin D3 conformers are illustrated in Fig. S1.†
Vitamin K
Vitamin K is a group of compounds with similar structures that act as post-translational modifiers of certain proteins involved in blood coagulation and metabolic pathways in bone and other tissues.33 Several subtypes of vitamin K2 with different isoprenoid chain lengths are observed. These homologues are called menaquinones and are characterized by the number of isoprenoid residues in their side chains; they are abbreviated to MK-n, where M stands for menaquinones, K stands for vitamin K and n represents the number of isoprenoid side chain residues. In this work, the photoelectron spectrum of MK-1 was calculated and assigned. There are twelve conformers of MK-1,34 and the frequency calculations show that only one of them is populated in the gas phase. The structure of this conformer is shown in Fig. 1, and its calculated photoelectron spectrum is shown in Fig. 4. It should be mentioned that an energy shift of approximately 1.5 eV is also expected for vitamin K because of the use of the direct SAC-CI calculation method. No theoretical and experimental study on the gas phase ionization energies and photoelectron spectrum of vitamin K2 has been reported in the literature. Only one investigation on the atmospheric pressure ionization and atmospheric pressure chemical ionization of menaquinones has been reported in the literature.35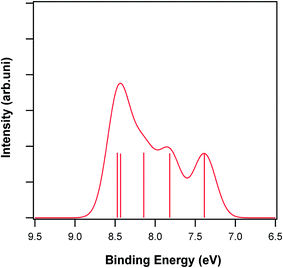 | ||
| Fig. 4 Calculated photoelectron spectrum of vitamin K (MK-1). Vertical lines show the calculated positions and intensities of ionization bands. | ||
The first ionization band of MK-1 is related to the ionization from HOMO, HOMO − 3 and HOMO − 4, and the contributions of HOMO and HOMO − 4 to the wave function are greater than that of HOMO − 3 (Table 1). This means that Koopmans' approximation is not valid for this vitamin. Based on the NBO calculations, the HOMO is a π molecular orbital that is mostly localized on the side chain C14![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C10 bond, whereas HOMO − 4 is a non-bonding orbital related to the lone electron pairs of the menaquinone oxygen atoms (Table S1 and Fig. S3†). Therefore, the ionization of MK-1 occurs from two different places in the molecule with the nearly same probability. Interestingly, the first ionization of this vitamin does not occur from the π electrons of the aromatic ring. The wave function of the second ionization band is a linear combination of three ionized HF determinants including HOMO − 1 (greatest contribution), HOMO − 2 and HOMO − 3. HOMO − 1 is a π molecular orbital localized on the aromatic ring (π(C13
C10 bond, whereas HOMO − 4 is a non-bonding orbital related to the lone electron pairs of the menaquinone oxygen atoms (Table S1 and Fig. S3†). Therefore, the ionization of MK-1 occurs from two different places in the molecule with the nearly same probability. Interestingly, the first ionization of this vitamin does not occur from the π electrons of the aromatic ring. The wave function of the second ionization band is a linear combination of three ionized HF determinants including HOMO − 1 (greatest contribution), HOMO − 2 and HOMO − 3. HOMO − 1 is a π molecular orbital localized on the aromatic ring (π(C13![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C16); π(C12
C16); π(C12![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C15); π*(C6
C15); π*(C6![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C7); see Fig. S3†); the ionization probability from π(C13
C7); see Fig. S3†); the ionization probability from π(C13![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C16) and π(C12
C16) and π(C12![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C15) is higher than that from π*(C6
C15) is higher than that from π*(C6![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C7). The third ionization band is related to HOMO − 2 (more contribution), HOMO − 3 and HOMO − 4 with different probabilities. HOMO − 2 is a π molecular orbital related to the π system of the aromatic ring and C3
C7). The third ionization band is related to HOMO − 2 (more contribution), HOMO − 3 and HOMO − 4 with different probabilities. HOMO − 2 is a π molecular orbital related to the π system of the aromatic ring and C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C4. HOMO − 3 is a π molecular orbital mostly related to the menaquinone C3
C4. HOMO − 3 is a π molecular orbital mostly related to the menaquinone C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C4 and localized primarily on this bond (Fig. S3†). The assignment of the fifth ionization band is similar to that of the first band because the wave function of this ionic state is a linear combination of two ionized determinants related to HOMO and HOMO − 4. Finally, it is important to note that the first ionization of vitamin K does not take place from the aromatic ring, but instead occurs from the unsaturated C14
C4 and localized primarily on this bond (Fig. S3†). The assignment of the fifth ionization band is similar to that of the first band because the wave function of this ionic state is a linear combination of two ionized determinants related to HOMO and HOMO − 4. Finally, it is important to note that the first ionization of vitamin K does not take place from the aromatic ring, but instead occurs from the unsaturated C14![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C10 bond of the side chain and the menaquinone oxygen atoms.
C10 bond of the side chain and the menaquinone oxygen atoms.
Vitamin E
Vitamin E is a group of vitamins including tocopherol and tocotrienol.36 In this work, the ionization energies of the tocopherol group were calculated. There are four tocopherol forms of vitamin E including α-, β-, γ- and δ-tocopherol. The most biologically active form of vitamin E is α-tocopherol.37 Fig. 5 shows the molecular structures of these four forms of vitamin E. The only experimental photoelectron spectrum of vitamin E in the literature was reported by Nagaoka et al.;15 they recorded the He–I photoelectron spectra of α-, β-, γ- and δ-tocopherol in the range of 8 to 21 eV. Fig. 6 and 7 show the calculated photoelectron spectra of the different forms of vitamin E and compare them with the corresponding experimental spectra recorded by Nagaoka et al.15 A good agreement between the calculated and experimental spectra is observed. It should be mentioned that the calculated spectra of α-tocopherol, β-tocopherol and tocol have been shifted towards higher binding energies by 1.6 eV in Fig. 6. Similarly, the calculated spectra of δ-tocopherol and γ-tocopherol have been shifted towards the higher binding energy by 1.5 and 1.7 eV, respectively. As seen in Fig. 6 and 7, the calculated five ionization bands produce three visible features in the calculated photoelectron spectra, with the exception of the α-tocopherol spectrum. Based on the calculations, the first ionization energies for the E vitamins increase in the following order: α-tocopherol < β-tocopherol < γ-tocopherol < δ-tocopherol < tocol (Table 2).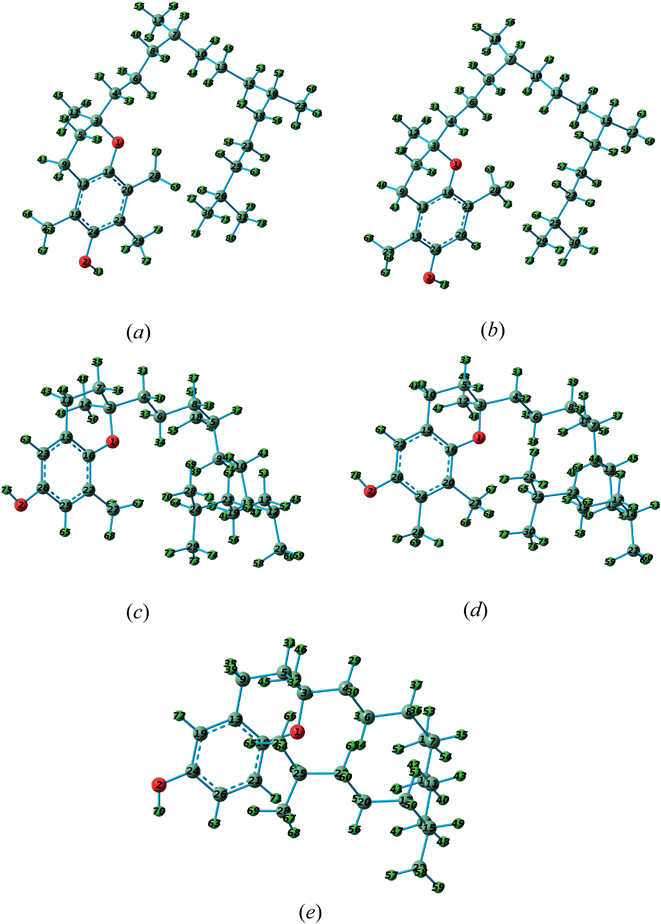 | ||
| Fig. 5 The molecular structures of different forms of vitamin E including (a) α-tocopherol, (b) β-tocopherol, (c) γ-tocopherol, (d) δ-tocopherol and (e) tocopherol. | ||
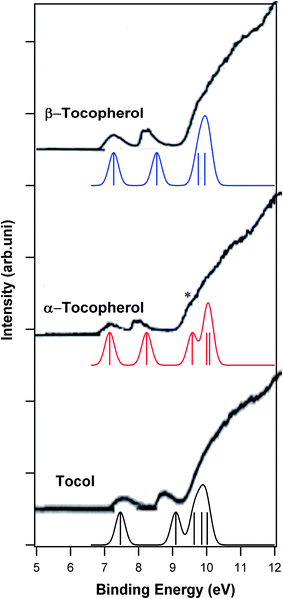 | ||
| Fig. 6 Comparison of the calculated photoelectron spectra of tocopherol, α-tocopherol and β-tocopherol with their experimental spectra.15 Vertical lines show the calculated positions and intensities of ionization bands. | ||
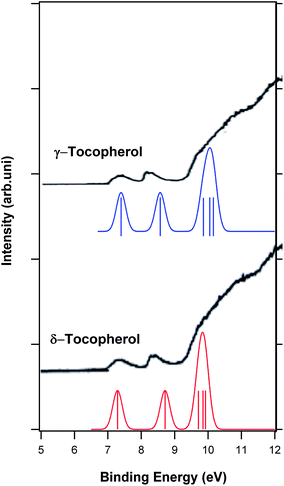 | ||
| Fig. 7 Comparison of the calculated photoelectron spectra of γ-tocopherol and δ-tocopherol with their experimental spectra.15 Vertical lines show the calculated positions and intensities of ionization bands. | ||
| State (2A) | Main electronic configuration | Ionization energy (eV) | Intensity | |
|---|---|---|---|---|
| Tocopherol | 1 | −0.93(HOMO) | 5.865 (7.465) | 0.912 |
| 2 | 0.95(HOMO − 1) | 7.494 (9.094) | 0.910 | |
| 3 | −0.91(HOMO − 2) | 8.035 (9.635) | 0.907 | |
| 4 | 0.93(HOMO − 3) | 8.256 (9.856) | 0.909 | |
| 5 | 0.94(HOMO − 4) | 8.416 (10.016) | 0.911 | |
| α-Tocopherol | 1 | −0.94(HOMO) | 5.550 (7.15) | 0.910 |
| 2 | 0.95(HOMO − 1) | 6.636 (8.236) | 0.911 | |
| 3 | −0.89(HOMO − 2) | 7.978 (9.578) | 0.895 | |
| 4 | −0.8(HOMO − 3) − 0.20(HOMO − 4) + 0.44(HOMO − 5) | 8.399 (9.999) | 0.901 | |
| 5 | −0.95(HOMO − 4) | 8.486 (10.086) | 0.911 | |
| β-Tocopherol | 1 | 0.93(HOMO) | 5.669 (7.269) | 0.912 |
| 2 | 0.94(HOMO − 1) | 6.934 (8.534) | 0.911 | |
| 3 | −0.77(HOMO − 2) + 0.40(HOMO − 4) | 8.154 (9.754) | 0.902 | |
| 4 | −0.91(HOMO − 2) − 0.20(HOMO − 3) | 8.347 (9.947) | 0.910 | |
| 5 | −0.81(HOMO − 4) − 0.460(HOMO − 2) | 8.457 (10.057) | 0.912 | |
| γ-Tocopherol | 1 | 0.94(HOMO) | 5.696 (7.396) | 0.911 |
| 2 | −0.95(HOMO − 1) | 6.868 (8.568) | 0.912 | |
| 3 | 0.94(HOMO − 2) | 8.163 (9.863) | 0.902 | |
| 4 | −0.72(HOMO − 3) + 0.59(HOMO − 4) | 8.350 (10.05) | 0.910 | |
| 5 | 0.76(HOMO − 4) + 0.59(HOMO − 3 | 8.459 (10.159) | 0.912 | |
| δ-Tocopherol | 1 | −0.94(HOMO) | 5.79 (7.29) | 0.912 |
| 2 | 0.95(HOMO − 1) | 7.213 (8.713) | 0.911 | |
| 3 | 0.92(HOMO − 2) | 8.213 (9.713) | 0.912 | |
| 4 | 0.8(HOMO − 3) − 0.45(HOMO − 4) | 8.348 (9.848) | 0.909 | |
| 5 | −0.20(HOMO − 6) + 0.77(HOMO − 4) + 0.45(HOMO − 3) | 8.423 (9.923) | 0.904 |
The first ionization bands of all forms of vitamin E are related to the ionization from HOMO. This molecular orbital is mostly localized on the aromatic ring (Fig. S2 and S3†) and related to two πC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and one π*C
C and one π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the ring (Table S2†). Table S2† also shows that there is a small probability for the ionization of vitamin E from the lone electron pairs of oxygen. Based on calculations using the HF/STO-3G method, Nagaoka et al.15 assigned the first ionization band of vitamin E only to the ionization of lone electron pairs of oxygen; in the present study, this ionization band was attributed primarily to the πC
C of the ring (Table S2†). Table S2† also shows that there is a small probability for the ionization of vitamin E from the lone electron pairs of oxygen. Based on calculations using the HF/STO-3G method, Nagaoka et al.15 assigned the first ionization band of vitamin E only to the ionization of lone electron pairs of oxygen; in the present study, this ionization band was attributed primarily to the πC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and π*C
C and π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the aromatic ring based on the SAC-CI calculation. The second ionization band of all forms of vitamin E is mainly related to the ionization of HOMO − 1 localized on the phenyl ring. HOMO − 1 is a π molecular orbital related to the π C
C of the aromatic ring based on the SAC-CI calculation. The second ionization band of all forms of vitamin E is mainly related to the ionization of HOMO − 1 localized on the phenyl ring. HOMO − 1 is a π molecular orbital related to the π C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the aromatic ring. The shapes of HOMO and HOMO − 1 for all forms of vitamin E are illustrated in Fig. S2 and S3.† Based on the above assignments, the first and second ionizations of vitamin E originate from the π electrons of the aromatic ring. The calculated spectra in Fig. 6 and 7 show that the energy difference between the first and second ionization band decreases from α-tocopherol, β-tocopherol, γ-tocopherol and δ-tocopherol compared to tocol. The third ionization band in the calculated spectrum of α-tocopherol is a resolved feature that has been identified with an asterisk in Fig. 6. The third ionization band is related to HOMO − 2, which is an σ orbital. The other molecular orbitals of all forms of vitamin E have σ character. Most importantly, the ionization of vitamin E from the lone electron pairs of oxygen does not take place below 10.5 eV; this conclusion is in contrast to the assignments made by Nagaoka et al.15
C of the aromatic ring. The shapes of HOMO and HOMO − 1 for all forms of vitamin E are illustrated in Fig. S2 and S3.† Based on the above assignments, the first and second ionizations of vitamin E originate from the π electrons of the aromatic ring. The calculated spectra in Fig. 6 and 7 show that the energy difference between the first and second ionization band decreases from α-tocopherol, β-tocopherol, γ-tocopherol and δ-tocopherol compared to tocol. The third ionization band in the calculated spectrum of α-tocopherol is a resolved feature that has been identified with an asterisk in Fig. 6. The third ionization band is related to HOMO − 2, which is an σ orbital. The other molecular orbitals of all forms of vitamin E have σ character. Most importantly, the ionization of vitamin E from the lone electron pairs of oxygen does not take place below 10.5 eV; this conclusion is in contrast to the assignments made by Nagaoka et al.15
Vitamin A
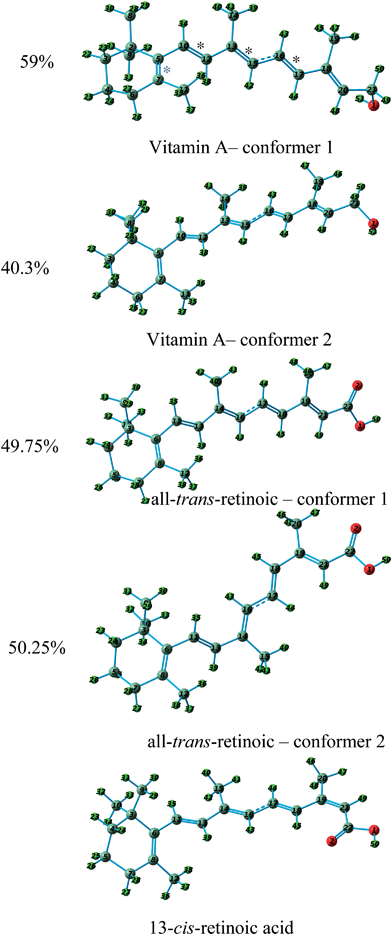
Vitamin A and its derivatives are very sensitive to light and heat because of the polyene skeleton present in their structures. Katsumata et al.20 studied the electronic structures of vitamin A and its derivatives using gas phase PES in order to understand their biological activity. They recorded the gas phase He–I photoelectron spectrum of vitamin A (all-trans-retinol and its two derivatives 13-cis-retinoic acid and all-trans-retinoic acid). In addition, they performed ab initio calculations at the HF/6-311G level of theory to calculate the ionization energy and molecular orbitals of each compound without considering the electron correlations. It should be mentioned that they assigned the photoelectron spectra using Koopmans' approximation. To date, no ab initio calculations have been reported on the ionization and photoelectron spectra of vitamin A and its derivatives considering the electron correlations.There are 30, 37 and 22 conformers for vitamin A, 13-cis-retinoic acid and all-trans-retinoic acid, respectively. The thermochemistry calculations performed in this work showed that only two conformers are populated in the gas phase for vitamin A and all-trans-retinoic acid, while only one conformer is populated for 13-cis-retinoic acid. The molecular structures of the conformers are depicted in Fig. 8. Fig. 9 shows the calculated photoelectron and BWP spectra of the vitamin A conformers and compares them with the experimental photoelectron spectrum of vitamin A.20 The calculated and experimental spectra of 13-cis-retinoic acid are also included in this figure. Fig. 10 shows the calculated photoelectron and BMP spectra of the conformers of all-trans-retinoic acid and compares them with its experimental spectrum.20
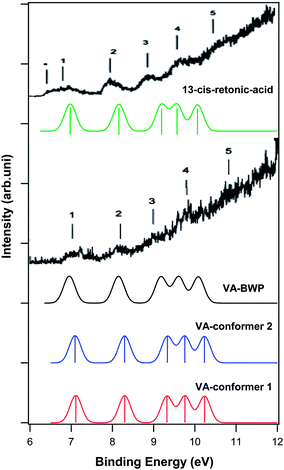 | ||
| Fig. 9 Comparison of the calculated photoelectron spectra of vitamin A conformers, their BWP spectrum and the calculated spectrum of 13-cis-retinoic acid with the experimental photoelectron spectra of vitamin A20 and 13-cis-retinoic acid.20 The numbers above the experimental spectra show the visible features identified by Katsumata et al.20 | ||
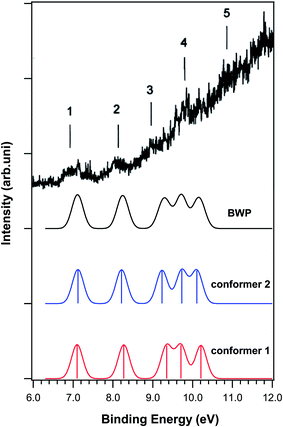 | ||
| Fig. 10 Comparison of the calculated photoelectron spectra and the BWP spectrum of the conformers of all-trans-retinoic acid with the experimental photoelectron spectrum of all-trans-retinoic acid.20 | ||
The calculated photoelectron spectra of the two conformers of vitamin A are very similar (Fig. 9). The calculated BWP spectrum of vitamin A describes its photoelectron spectra20 very well. Unfortunately, the resolution of the experimental spectrum17 is poor, however, the features can still be made out. The calculated photoelectron spectra of the conformers of all-trans-retinoic acid (Fig. 10) and 13-cis-retinoic acid are very similar to those of vitamin A. This means that the calculated ionization bands originate from the same region of the molecule in all three compounds. The calculated BWP spectra of vitamin A (1.5 eV) and all-trans-retinoic acid (1.3 eV) along with the spectrum of 13-cis-retinoic acid (1.25 eV) were shifted to higher binding energies to obtain the best agreement with the experiment. The main configuration of the ionic wave functions of vitamin A, all-trans-retinoic acid and 13-cis-retinoic acid obtained from SAC-CI calculations (Table 3) shows that the electronic correlations are not negligible in the calculation of ionization energies in the energy region considered; this is because the wave function of some ionic states are a linear combination of two or more single HF-ionized determinants. To see the effect of the correlation energy on the relative energy positions of the ionization bands, the calculated ionization energies of the vitamin A conformers relative to the energy of the first ionization band were plotted against the ionization band number (Fig. 11). There is a considerable difference between the plot obtained using HF theory (without electron correlation) and that obtained using SAC-CI theory. The plot obtained using the calculated ionization energies of Katsumata et al.20 has also been included in this figure. The electronic correlations are absent in the calculations of Katsumata et al., as shown by the similarity of their plot to those obtained in this work using the HF/D95(df,pd) method. The comparison of theory with experimental work in Fig. 9 and 10 again confirms that although the calculated photoelectron spectra were shifted to higher binding energies, the direct SAC-CI method can provide accurate predictions of the relative energies of ionization bands.
| State (2A) | Main electronic configuration | Ionization energy (eV) | Intensity | ||
|---|---|---|---|---|---|
| Vitamin A | Conformer I | 1 | −0.94(HOMO) | 5.611 (7.111) | 0.91 |
| 2 | −0.92(HOMO − 1) | 6.798 (8.298) | 0.904 | ||
| 3 | −0.87(HOMO − 2) − 0.25(HOMO − 3) | 7.823 (9.323) | 0.889 | ||
| 4 | −0.72(HOMO − 3) − 0.43(HOMO − 4) | 8.260 (9.76) | 0.893 | ||
| 5 | 0.22(HOMO − 2) − 0.38(HOMO − 3) − 0.81(HOMO − 4) | 8.734 (10.234) | 0.884 | ||
| Conformer II | 1 | −0.94(HOMO) | 5.594 (7.094) | 0.910 | |
| 2 | 0.92(HOMO − 1) | 6.796 (8.296) | 0.903 | ||
| 3 | 0.88(HOMO − 2) + 0.24(HOMO − 3) | 7.835 (9.335) | 0.888 | ||
| 4 | −0.72(HOMO − 3) + 0.44(HOMO − 4) | 8.255 (9.755) | 0.894 | ||
| 5 | −0.20(HOMO − 2) − 0.39(HOMO − 3) + 0.81(HOMO − 4) | 8.733 (10.233) | 0.883 | ||
| All-trans-retinoic acid | Conformer I | 1 | −0.93(HOMO) | 5.800 (7.1) | 0.905 |
| 2 | −0.92(HOMO − 1) | 6.973 (8.273) | 0.898 | ||
| 3 | 0.85(HOMO − 2) + 0.31(HOMO − 3) | 8.049 (9.349) | 0.882 | ||
| 4 | −0.64(HOMO − 3) − 0.45(HOMO − 6) | 8.407 (9.707) | 0.890 | ||
| 5 | −0.28(HOMO − 2) −0.38(HOMO − 3) − 0.79(HOMO − 4) | 8.909 (10.209) | 0.884 | ||
| Conformer II | 1 | 0.94(HOMO) | 5.822 (7.122) | 0.90662 | |
| 2 | 0.93(HOMO − 1) | 6.917 (8.217) | 0.90185 | ||
| 3 | −0.90(HOMO − 2) | 7.933 (9.233) | 0.88685 | ||
| 4 | −0.82(HOMO − 3) + 0.30(HOMO − 6) | 8.428 (9.728) | 0.89329 | ||
| 5 | −0.89(HOMO − 4) − 0.21(HOMO − 3) | 8.806 (10.106) | 0.88183 | ||
| 13-cis-Retinoic | 1 | 0.93(HOMO) | 5.726 (6.976) | 0.906 | |
| 2 | 0.92(HOMO − 1) | 6.908 (8.158) | 0.899 | ||
| 3 | −0.83(HOMO − 2) − 0.32(HOMO − 3) | 7.943 (9.193) | 0.882 | ||
| 4 | 0.68(HOMO − 3) − 0.49(HOMO − 6) | 8.316 (9.566) | 0.889 | ||
| 5 | 0.33(HOMO − 2) − 0.41(HOMO − 3) − 0.76(HOMO − 4) | 8.8139 (10.063) | 0.878 | ||
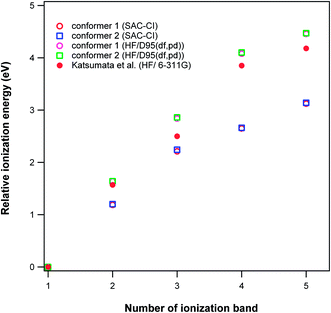 | ||
| Fig. 11 Variation in the energy positions of the ionization bands of vitamin A conformers relative to their first ionization bands versus the ionization band number. | ||
The first and second ionization bands of vitamin A, all-trans-retinoic acid and 13-cis-retinoic acid correspond to HOMO and HOMO − 1, respectively (Table 3). HOMO is a π molecular orbital related to five conjugate πC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C but primarily localized on three C
C but primarily localized on three C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds (identified with black asterisks in Fig. 8). Similarly, HOMO − 1 is a π molecular orbital, but it is mostly concentrated on only one C
C bonds (identified with black asterisks in Fig. 8). Similarly, HOMO − 1 is a π molecular orbital, but it is mostly concentrated on only one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (identified with a blue asterisk in Fig. 8; see also Table S3†). The shape of the molecular orbitals of vitamin A, all-trans-retinoic acid and 13-cis-retinoic acid are shown in Fig. S4 and S5.† The wave function of the third ionization band of the vitamin A conformers, 13-cis-retinoic acid and conformer 1 of all-trans-retinoic acid is a linear combination of two single HF-ionized determinants including HOMO − 2 (major contribution) and HOMO − 3. The ionic wave function of conformer 2 of all-trans-retinoic acid mainly originates from HOMO − 2. The third feature in the experimental spectra of these compounds corresponds to the third ionization band of each conformer. HOMO − 2 is a π molecular orbital mostly concentrated on one C
C bond (identified with a blue asterisk in Fig. 8; see also Table S3†). The shape of the molecular orbitals of vitamin A, all-trans-retinoic acid and 13-cis-retinoic acid are shown in Fig. S4 and S5.† The wave function of the third ionization band of the vitamin A conformers, 13-cis-retinoic acid and conformer 1 of all-trans-retinoic acid is a linear combination of two single HF-ionized determinants including HOMO − 2 (major contribution) and HOMO − 3. The ionic wave function of conformer 2 of all-trans-retinoic acid mainly originates from HOMO − 2. The third feature in the experimental spectra of these compounds corresponds to the third ionization band of each conformer. HOMO − 2 is a π molecular orbital mostly concentrated on one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (Table S3†). For example, this C
C bond (Table S3†). For example, this C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, for conformer 1 of vitamin A is C18
C bond, for conformer 1 of vitamin A is C18![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C20, which has a π contribution in HOMO − 2 of approximately 30.3%. Katsumata et al.20 proposed that the fourth feature of the experimental photoelectron spectra of vitamin A and its derivatives are composed of the fourth and fifth ionization bands based on the assignment using Koopmans' approximation. The SAC-CI calculations showed that the fourth feature in the experimental spectrum is composed of only the fourth ionization band. The reason for this discrepancy is related to the absence of electron correlations in the calculations of Katsumata et al.20 The wave function of the fourth ionic state of vitamin A is a linear combination of three single ionized HF determinants including HOMO − 3 and HOMO − 4 (major contribution; Table 3). The main configuration of the fourth ionic states of all-trans-retinoic acid and 13-cis-retinoic acid is a linear combination of HOMO − 3 and HOMO − 6. The assignment of the fourth ionization band of vitamin A is different from all-trans-retinoic acid and 13-cis-retinoic acid. HOMO − 3 is related to the chain of five πC
C20, which has a π contribution in HOMO − 2 of approximately 30.3%. Katsumata et al.20 proposed that the fourth feature of the experimental photoelectron spectra of vitamin A and its derivatives are composed of the fourth and fifth ionization bands based on the assignment using Koopmans' approximation. The SAC-CI calculations showed that the fourth feature in the experimental spectrum is composed of only the fourth ionization band. The reason for this discrepancy is related to the absence of electron correlations in the calculations of Katsumata et al.20 The wave function of the fourth ionic state of vitamin A is a linear combination of three single ionized HF determinants including HOMO − 3 and HOMO − 4 (major contribution; Table 3). The main configuration of the fourth ionic states of all-trans-retinoic acid and 13-cis-retinoic acid is a linear combination of HOMO − 3 and HOMO − 6. The assignment of the fourth ionization band of vitamin A is different from all-trans-retinoic acid and 13-cis-retinoic acid. HOMO − 3 is related to the chain of five πC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C for all compounds, while HOMO − 4 is related to the σC–C of the cyclohexene ring. The position of the fifth ionization band of vitamin A identified by Katsumata et al.20 in their experimental spectra (see Fig. 8 and 9) is not confirmed by the calculated spectra in this work. For example, our theoretical calculations show that the fifth ionization band of all-trans-retinoic is around 10.2 eV, while Katsumata et al.20 considered this feature to be around 10.8 eV, containing three ionization bands (the fifth, sixth and seventh). Finally, the theoretical calculations show that the ionization of vitamin A and its derivatives from the lone electron pairs of oxygen does not take place below 11 eV.
C for all compounds, while HOMO − 4 is related to the σC–C of the cyclohexene ring. The position of the fifth ionization band of vitamin A identified by Katsumata et al.20 in their experimental spectra (see Fig. 8 and 9) is not confirmed by the calculated spectra in this work. For example, our theoretical calculations show that the fifth ionization band of all-trans-retinoic is around 10.2 eV, while Katsumata et al.20 considered this feature to be around 10.8 eV, containing three ionization bands (the fifth, sixth and seventh). Finally, the theoretical calculations show that the ionization of vitamin A and its derivatives from the lone electron pairs of oxygen does not take place below 11 eV.
Conclusion
The ionization energies and valence photoelectron spectra of different vitamins including A, K, E and D were calculated using the direct SAC-CI method. The present calculations accurately simulated the shape of the experimental spectra and provided detailed assignments of the spectra. An energy shift was required to match the calculated spectra with the experimental spectra. This energy shift was attributed primarily to the intrinsic error present in the direct SAC-CI method, which increases with the size of the basis set and is partly due to the incompleteness of the basis set. Such an energy shift can be seen in the calculation of the near edge X-ray photo absorption spectra for guanine reported by Plekan et al. using the algebraic-diagrammatic construction approximation and the 6-31G basis set.38,39 However, we showed that the present direct SAC-CI SD-R method in combination with the D95(df,pd) basis set can predict the relative energies and intensities of the photoelectron lines of the compounds considered in this work quite accurately.Acknowledgements
The authors thank Isfahan University of Technology (IUT) for financial support and the national high performance computing center of IUT.References
- M. H. Stipanuk and M. A. Caudill, Biochemical, Physiological, and Molecular Aspects of Human Nutrition, 2013 Search PubMed.
- S. A. S. Gropper, J. L. Smith and J. L Groff, Advanced nutrition and human metabolism, Wadsworth/Cengage Learning, Australia, USA, 2009 Search PubMed.
- S. A. Tanumihardjo, Am. J. Clin. Nutr., 2011, 94, 658S–665S CrossRef PubMed.
- R. L. Lundblad, Biochemistry and Molecular Biology Compendium, Taylor & Francis, 2007 Search PubMed.
- I. Novak and A. W. Potts, Biochim. Biophys. Acta, Bioenerg., 1997, 1319, 86–90 CrossRef CAS.
- T. Brody, Nutritional Biochemistry, Academic Press, New York, 1994 Search PubMed.
- C. Vermeer, L. Braam, M. Knapen and L. Schurgers, Agro Food Ind. Hi-Tech, 2003, 14, 17–20 Search PubMed.
- D. A. Bender, Introduction to Nutrition and Metabolism, Taylor & Francis, 2014 Search PubMed.
- E. Nachbar and K. E. Nachbar, J. Mol. Med., 1995, 73, 7–17 CrossRef.
- T. Koutchma, L. J. Forney and C. I. Moraru, Ultraviolet Light in Food Technology: Principles and Applications, Taylor & Francis, 2010, p. 107 Search PubMed.
- V. E. Kagan, A. Shvedova, E. Serbinova, S. Khan, C. Swanson, R. Powell and L. Packer, Biochem. Pharmacol., 1992, 44, 1637–1649 CrossRef CAS.
- H. Farrokhpour and M. Ghandehari, J. Phys. Chem. B, 2013, 117, 6027–6041 CrossRef CAS PubMed.
- D. Ghosh, O. Isayev, L. V. Slipchenko and A. I. Krylov, J. Phys. Chem. A, 2011, 115, 6028–6038 CrossRef CAS PubMed.
- P. Slavicek, B. Winter, M. Faubel, S. E. Bradforth and P. Jungwrith, J. Am. Chem. Soc., 2009, 131, 6460–6467 CrossRef CAS PubMed.
- S. I. Nagaoka, K. Mukai, T. Itoh and S. Katsumata, J. Phys. Chem., 1992, 96, 8184–8187 CrossRef CAS.
- J. E. Šponer, V. Sychrovský, P. Hobza and J. Šponer, Phys. Chem. Chem. Phys., 2004, 6, 2772–2780 RSC.
- M. Segala, Y. Takahata and D. P Chong, J. Mol. Struct., 2006, 758, 61–69 CAS.
- S. Saha, F. Wang, J. B. MacNaughton, A. Moewes and D. P. Chong, J. Synch. Rad, 2008, 15, 151–157 CrossRef CAS PubMed.
- Z. Jericevic, L. Klasinc, B. Kovac and I. Novak, Kem. Ind., 1980, 29, 117 CAS.
- S. Katsumata and N. Ikehata, J. Electron Spectrosc. Relat. Phenom., 2000, 107, 139–145 CrossRef CAS.
- H. Nakatsuji, O. Kitao and T. Yonezawa, J. Chem. Phys., 1985, 83, 723–734 CrossRef CAS PubMed.
- M. Ehara and H. Nakatsuji, Chem. Phys. Lett., 1998, 282, 347–354 CrossRef CAS.
- H. Nakatsuji, M. Ehara, M. H. Palmer and M. F. Guest, J. Chem. Phys., 1992, 97, 2561–2570 CrossRef CAS PubMed.
- H. Nakatsuji and M. Ehara, J. Chem. Phys., 1994, 101, 7658–7671 CrossRef CAS PubMed.
- H. Nakatsuji, J. Hasegawa and M. Hada, J. Chem. Phys., 1996, 104, 2321–2330 CrossRef CAS PubMed.
- M. Ehara, Y. Ohtsuka and H. Nakatsuji, J. Chem. Phys., 1998, 226, 113–123 CAS.
- H. Nakatsuji and S. Saito, J. Chem. Phys., 1989, 91, 6205–6214 CrossRef CAS PubMed.
- H. Farrokhpour and F. Fathi, J. Comput. Chem., 2011, 32, 2479–2491 CrossRef CAS PubMed.
- R. L. Martin and D. A. Shirley, Chem. Phys., 1976, 64, 3685–3689 CAS.
- E. D. Glendening, J. K. Badenhoop, A. E. Read, J. E. Carpenter, J. A. Bohmann, C. M. Morales and F. Weinhold, NBO Version 5.0, Theoretical Chemistry Institute, University of Wisconsin, Madison, 2001 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. KeithR. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- R. Fukuda and H. Nakatsuji, J. Chem. Phys., 2008, 128, 094105 CrossRef PubMed.
- F. Borrelli and E. Ernst, Maturitas, 2010, 66, 333–343 CrossRef PubMed.
- K. Nimptsch, S. Rohrmann and J. Linseisen, Am. J. Clin. Nutr., 2008, 87, 985–992 CAS.
- R. Geyer, A. D. Peacock, D. C. White, C. Lytle and G. J. Van Berkel, J. Mass Spectrom., 2004, 39, 922–929 CrossRef CAS PubMed.
- F. Aguilar, H. Autrup, S. Barlow, L. Castle, R. Crebelli, W. Dekant, K. H. Engel, N. Gontard, D. Gott, S. Grilli, R. Gürtler, J. C. Larsen, C. Leclercq, J. C. Leblanc, X. Malcata, W. Mennes, M. R. Milana, I. Pratt, I. Rietjens, P. Tobback and F. Toldrá, EFSA J., 2008, 640, 1–34 Search PubMed.
- R. Brigelius-Flohé and M. G. Traber, FASEB J., 1999, 13, 1145–1155 Search PubMed.
- Y. Li, J. Wan and X. Xu, J. Comput. Chem., 2007, 28, 1667.31 Search PubMed.
- O. Plekan, V. Feyer, R. Richter, M. Coreno, G. Vall-llosera, K. C. Prince, A. B. Trofimov, I. L. Zaytseva, T. E. Moskovskaya, E. V. Gromov and J. Schirmer, J. Phys. Chem. A, 2009, 113, 9376 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05271d |
| This journal is © The Royal Society of Chemistry 2014 |