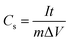Facile synthesis and high electrochemical performance of porous carbon composites for supercapacitors
Jiangfeng Lia,
Qingsheng Wu*abc and
Guangtao Zanc
aDepartment of Chemistry, Key Laboratory of Yangtza River Water Environment, Ministry of Education, Tongji University, Shanghai 200092, PR China. E-mail: qswu@tongji.edu.cn; Fax: +86 21 65981097; Tel: +86 21 65982620
bShanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Fudan University, Shanghai 200433, PR China
cSchool of Materials Science and Engineering, Tongji University, Shanghai 200092, PR China
First published on 31st July 2014
Abstract
A facile and green method was used to synthesize Fe2O3/mushroom derived porous carbon (Fe2O3/MPC) nanocomposites with high capacitive properties. The resultant materials were analyzed by scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), transmission electron microscopy (TEM), powder X-ray diffraction (XRD), and Brunauer–Emmett–Teller (BET) measurements. It is shown by the BET surface area measurements that MPC has a high specific surface area and abundant porosity, which are conducive to improve the electrochemical performance. The Fe2O3/MPC nanocomposite exhibits a maximum specific capacitance of 367.39 F g−1 at a current density of 0.5 A g−1 and good capacitance stability over 1500 cycles at a current density of 5 A g−1. These attractive results indicate that the Fe2O3/MPC nanocomposite could be a potential electrode material for supercapacitors.
1 Introduction
Increasing energy demands and uncertainties in energy supply urge researchers to develop efficient energy storage systems. Supercapacitors are becoming attractive power sources because of their excellent potential performance. As energy storage devices, supercapacitors can be classified into electrical double-layer capacitors (EDLCs) and pseudocapacitors based on their underlying charge-storage mechanism.1 Compared with conventional capacitors, supercapacitors have been more widely concerned owing to their high power density, short charging time and long cycling life.2–5 However, the practical applications of supercapacitors are still limited because of unsatisfactory performance of the electrode materials and the high production cost of noble-metal oxides based materials.6,7 Therefore, the main challenge for researchers is to develop high performance and low-cost materials.Recent studies are shown that activated carbons are suitable candidates for EDLCs on account of their high surface area, excellent electrochemical stability, good electrical conductive properties, etc.8 However, conventional carbon precursors like fossil fuels of petroleum coke9 and coal tar pitches10 are not the ideal materials for supercapacitors on account of their unsustainability and non-renewable. Recently, the use of biological materials as precursors for activated carbons has been attracting researchers' attention because of low cost, accessibility, renewable, porous structures and complex morphologies.11,12 Most biological materials have porous structures, such as sugarcane bagasse,13 fish scale,14 neem leaves,15 peanut shell,16 etc. But the flaw in EDLCs shows low energy density and capacitance. To overcome this problem, researches are focused on introducing transitional metal oxides (e.g., RuO2, MnO2, NiO, Fe2O3, Cu2O) to porous carbon materials. These transitional metal oxides can exhibit large capacitance and high energy density due to their reversible multielectron redox faradaic reactions.17 Comparing with the other transitional metal oxides, Fe2O3 has higher theoretical capacitance, lower production costs and abundance.18–20 So it has become a suitable material for supercapacitors. However, two main shortcomings limit its application: low conductivity and rapid capacity decay.21,22
Mushroom, a kind of readily available goods in the market, has porous structures observed in the scanning electron microscopy (SEM) analysis, may be an excellent biological materials for developing supercapacitors. In this study, we report a facile and green method to fabricate Fe2O3/MPC nanocomposite from iron nitrate and inexpensive mushroom derived porous carbon material for supercapacitors via a simple calcination method under gas protection.
2 Experimental section
2.1 Materials
The beech mushrooms were purchased from market in Shanghai. Potassium hydroxide, ethanol, hydrochloric acid, nitric acid, sulfuric acid, graphite powder were purchased from Shanghai Chemical Reagents Co., Ltd. Polytetrafluoroethylene (PTFE) solution was purchased from Shanghai 3F New Materials Co., Ltd. Nickel foil was purchased from Shanghai Hongxiang Plant. Pure nitrogen was purchased from Shanghai BOC Special Gases Sales Service Co., Ltd. Deionized water was used throughout the experiments.2.2 Preparation of Fe2O3/MPC composite
The mushrooms were washed with deionized water and dried at 80 °C. The cleaned beech mushrooms were subsequently pre-carbonized at 500 °C for 2 h under argon atmosphere. Then the pre-carbonized materials were ground to powder, mixed with KOH (mKOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mc = 2), ultrasonic treatment for 30 min, and dried at 120 °C for 12 h. After that, the mixtures were heated in a ceramic crucible from room temperature to 800 °C at a rate of 5 °C min−1 and the final temperature was kept for 2 h under argon atmosphere. Finally, the dark products were washed with excess 1 M HCl solution and then washed several times with deionized water. The remainders were dried at 80 °C to obtain the final product of MPC.
mc = 2), ultrasonic treatment for 30 min, and dried at 120 °C for 12 h. After that, the mixtures were heated in a ceramic crucible from room temperature to 800 °C at a rate of 5 °C min−1 and the final temperature was kept for 2 h under argon atmosphere. Finally, the dark products were washed with excess 1 M HCl solution and then washed several times with deionized water. The remainders were dried at 80 °C to obtain the final product of MPC.
0.5 g MPC was impregnated with 50 mL Fe(NO3)3·9H2O solution. After ultrasonic treatment, the mixture was keeping at room temperature for 6 h. Then, the resultant solution was filtered, and the samples were dried at 60 °C. The obtained powders were annealed at 800 °C for 3 h without oxygen to get the final product of Fe2O3/MPC nanocomposite.
2.3 Preparation of the electrodes
The working electrode was prepared by mixing the composite (85 wt%), graphite power (10 wt%) and PTFE (5 wt%), using deionized water as solvent. Then, the slurry was applied to a nickel foam (1 cm × 1 cm) at 6 MPa and dried at 80 °C. Electrochemical measurements were performed with a three electrode experimental setup, nickel foam and Ag–AgCl electrode were used as the counter and reference electrodes in a 6 M KOH solution, respectively.2.4 Characterization techniques
Scanning electron microscopy (SEM, Phenom Pro-G2, Netherlands), energy dispersive spectroscope (EDS) and transmission electron microscopy (TEM, JEOL JEM-2100, Japan) were used to characterize the surface morphology and the elemental compositions of the Fe2O3/MPC nanocomposites. The adsorption amounts were obtained from the Micromeritics Tristar 3000 gas adsorption analyzer. The specific surface area was calculated by using a single-point Brunauer–Emmett–Teller (BET) method. Thermogravimetric analysis was carried out on a NETZSCH STA409 PC thermal analysis instrument, and the heating rate was 5 °C min−1. Powder X-ray diffraction (XRD) patterns were performed on Bruker Focus D8 diffractometer with Cu Kα radiation (40 kV, λ = 0.15418 nm) between 5 and 70°. Electrochemical measurements were tested by a CHI 660E electrochemical workstation (ChenHua Corp., Shanghai, China).3 Results and discussion
3.1 Sample characterization
Scheme 1 illustrates a schematic diagram of formation process of MPC and Fe2O3/MPC nanocomposite. The fresh mushroom was pre-carbonized at 500 °C, and further carbonized at 800 °C with KOH as activating agent. After the above steps, there are abundant micropores inside mushroom-derived porous carbon, which provide a place to store iron nitrate solution. During the drying and calcination processes, iron nitrate finally decomposed into ferric oxide and formed the Fe2O3/MPC nanocomposite.The surface morphology and structure of the prepared materials were examined using SEM and TEM techniques. Fig. 1A shows that the surface of carbonized mushroom has lax and porous structure. The abundant micropores are formed due to KOH activation in pre-carbonized mushroom (Fig. 1B). Fig. 1C and D show the morphology of Fe2O3 nanoparticles anchored on MPC and the uniform dispersion of Fe2O3 nanoparticles. The synthesis of Fe2O3 on MPC can be inferred as follows:
Fig. 2A shows the chemical compositions of MPC are consist of C and O, which indicates the MPC materials do not have other impurity elements. Two weak peaks are observed in Fig. 4B which confirm the existence of a small amount iron in Fe2O3/MPC nanocomposite. The EDS result proves that the experimental method of this paper is effective in loading iron element on MPC.
Fig. 3A shows the XRD patterns of MPC and Fe2O3/MPC. It is distinctly that MPC material possess a typical graphitic stacking peak around at 22°, which should enhance the electrochemical performance.23,24 For sample Fe2O3/MPC, the characteristic peaks such as (012), (104), (110), (113), (024) (116) (214) and (300) can be indexed to Fe2O3 (JCPDS File no. 33-0664). The peak related to carbon can not be observed in the diffraction patterns, which means that the carbon is present in the composite in the amorphous state.25 It is entirely consistent with the previous result of EDS analysis. Fig. 3B shows a thermogravimetric curve of the sample Fe2O3/MPC in the air. The weight loss was 6% between 20 and 250 °C, because of the evaporation of physically absorbed water. There is no more weight loss at more than 600 °C, and the remains are Fe2O3 nanoparticles (about 68%). The result indicates that the amount of Fe2O3 nanoparticles is 72% in the nanocomposites.
 | ||
| Fig. 3 (A) X-Ray diffraction patterns of MPC and Fe2O3/MPC; (B) thermogravimetric curve of Fe2O3/MPC. | ||
In order to obtain the surface area and pore size distribution of the as-prepared Fe2O3/MPC nanocomposites, the liquid nitrogen cryosorption measurements were conducted. Fig. 4A shows the nitrogen adsorption–desorption isotherms of two samples could be type I characteristics with a H4 hysteresis loop,26 indicating the typical microporous character of the samples.27,28 Pore size distribution of the samples is shown in Fig. 4B, which shows that synthesized samples have the similar pore size distributions in the range of 2–5 nm. The specific porous properties of the samples are detailed in Table 1. The specific surface area of MPC and Fe2O3/MPC are 1639.04 and 1361.98 m2 g−1 with the volumes of 0.83 and 0.69 cm3 g−1, respectively. Moreover, the high specific surface area and abundant porosity of sample MPC facilitates effective access of electrolyte ions to the electrode surfaces, which are conducive to improve the electrochemical properties of supercapacitors.29 As displayed in Table 1, the micropore volume (Vmicro) are 0.76 and 0.63 (cm3 g−1) for MPC and Fe2O3/MPC, respectively. Furthermore, the specific surface area and pore volume of micropore decreased slightly after Fe2O3 nanoparticles filled into the micropores of the MPC.
 | ||
| Fig. 4 (A) N2 adsorption–desorption isotherms and (B) pore-size distributions of samples MPC and Fe2O3/MPC. | ||
| Samples | SBET [m2 g−1] | Smico [m2 g−1] | Smeso [m2 g−1] | SLangmuir [m2 g−1] | Vpore [cm3 g−1] | Vmicro [cm3 g−1] | Vmeso [cm3 g−1] | Daver [nm] |
|---|---|---|---|---|---|---|---|---|
| MPC | 1639.04 | 1604.27 | 34.77 | 2253.52 | 0.83 | 0.76 | 0.07 | 2.03 |
| Fe2O3/MPC | 1361.98 | 1335.31 | 26.67 | 1867.68 | 0.69 | 0.63 | 0.06 | 2.03 |
3.2 Electrochemical properties
where Cs is the specific capacitances (F g−1), I is the response current (A), ν is the scan rate (mV s−1), ΔV is the potential window (V), and m is the mass of the electrode (g). According to the above formula, the specific capacitances of MPC and Fe2O3/MPC are found to be 268.74 and 171.51 F g−1 at 1 mV s−1, respectively. Fig. 5C lists all specific capacitances with different scan rate for MPC and Fe2O3/MPC. In present study, the capacitance of Fe2O3/MPC is much higher than a-Fe2O3/multi-walled carbon nanotubes32 and graphene/Fe2O3,33 reported previously. The high electrochemical performance of Fe2OS3/MPC could be due to the high specific surface area with abundant porosity and Fe2O3 loading effects.
where Cs is the specific capacitance (F g−1), I is the discharge current (A), t is the discharge time (s), m is the active materials, ΔV is the potential difference (V). As described in Fig. 6, the discharge time of Fe2O3/MPC much longer than MPC at the same current density owing to the pseudocapacitance of Fe2O3. Table 2 shows the specific capacitance at different current densities ranged from 0.5 to 5 A g−1. When the current density at 0.5 A g−1, Fe2O3/MPC exhibits the specific capacitance of 367.39 F g−1, which means the existence of Fe2O3 nanoparticles is effective to improve the specific capacitance of carbon material. As listed in Table 2, Fe2O3/MPC still remained specific capacitance of 213.94 F g−1 at a current density of 5 A g−1. But with the increase of current density, the specific capacitance of Fe2O3/MPC dropped apparently. This trend of the capacitance illustrates that the redox reaction can not keep high rate at a high current density.34
| Electrode | Current density | |||||
|---|---|---|---|---|---|---|
| 0.5 A g−1 | 1 A g−1 | 2 A g−1 | 3 A g−1 | 4 A g−1 | 5 A g−1 | |
| MPC (F g−1) | 199.36 | 177.94 | 167.60 | 163.68 | 161.40 | 160.13 |
| Fe2O3/MPC (F g−1) | 367.39 | 290.13 | 248.44 | 235.18 | 221.95 | 213.94 |
4 Conclusion
Fe2O3/MPC nanocomposite for supercapacitors was successfully synthesized through a facile and green method. Owing to the loading of Fe2O3, the specific capacitance of Fe2O3/MPC nanocomposite is greatly improved compared with MPC. The Fe2O3/MPC electrode material exhibits the maximum specific capacitance of 367.39 F g−1 at a current density of 0.5 A g−1 and good capacitance stability (82.7%) over 1500 cycles at a current density of 5 A g−1. The results indicate that the Fe2O3/MPC nanocomposite is a potential ideal material for supercapacitors.Acknowledgements
The authors are grateful to the financial support of the National Natural Science Foundation of China (no. 91122025, 21103127, 21101118), the State Major Research Plan (973) of China (no. 2011CB932404), the Nano-Foundation of Shanghai in China (no. 11nm0501300), the State Key Laboratory of Fine Chemicals (no. KF1103) and the Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials (no. 2012MCIMKF03).References
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- A. Ghosh and Y. H. Lee, ChemSusChem, 2012, 5, 480–499 CrossRef CAS PubMed.
- C. Merlet, B. Rotenberg, P. A. Madden, P. L. Taberna, P. Simon, Y. Gogotsi and M. Salanne, Nat. Mater., 2012, 11, 306–310 CrossRef CAS PubMed.
- C. Guan, X. Xia, N. Meng, Z. Zeng, X. Cao, C. Soci, H. Zhang and H. J. Fan, Energy Environ. Sci., 2012, 5, 9085–9090 Search PubMed.
- H. Jiang, P. S. Lee and C. Z. Li, Energy Environ. Sci., 2013, 6, 41–53 Search PubMed.
- F. Deng, L. Yu, G. Cheng, T. Lin, M. Sun, F. Ye and Y. Li, J. Power Sources, 2014, 251, 202–207 CrossRef CAS PubMed.
- G. P. Wang, L. Zhang and J. J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 Search PubMed.
- M. Biswal, A. Banerjee, M. Deo and S. B. Ogale, Energy Environ. Sci., 2013, 6, 1249–1259 CAS.
- K. Xu, Y. Li, F. Yang, W. Yang, L. Zhang, C. Xu, T. Kaneko and R. Hatakeyama, Carbon, 2014, 68, 511–519 CrossRef CAS PubMed.
- C. Zeng, Q. Lin, C. Fang, D. Xu and Z. Ma, J. Anal. Appl. Pyrolysis, 2013, 104, 372–377 CrossRef CAS PubMed.
- S. T. Senthilkumar, R. Kalai Selvan, J. S. Melo and C. Sanjeeviraja, ACS Appl. Mater. Interfaces, 2013, 5, 10541–10550 CAS.
- F. Qu, H. Lin, X. Wu, X. Li, S. Qiu and G. Zhu, Solid State Sci., 2010, 12, 851–856 CrossRef CAS PubMed.
- J. Pan, X. Cheng, X. Yan and C. Zhang, J. Eur. Ceram. Soc., 2013, 33, 575–581 CrossRef CAS PubMed.
- W. Chen, H. Zhang, Y. Huang and W. Wang, J. Mater. Chem., 2010, 20, 4773–4775 Search PubMed.
- M. Biswal, A. Banerjee, M. Deo and S. Ogale, Energy Environ. Sci., 2013, 6, 1249–1259 Search PubMed.
- X. He, K. Xie, R. Li and M. Wu, Mater. Lett., 2014, 115, 96–99 Search PubMed.
- J. Chen, K. Huang and S. Liu, Electrochim. Acta, 2009, 193, 935–938 Search PubMed.
- Y. Li, Y. Lu, H. Hong, Y. Chen, X. Ma, L. Guo, Z. Wang, J. Chen, M. Zhu, J. Ni, H. Gu, J. Lu and J. Y. Ying, Chem. Commun., 2011, 47, 6320–6322 RSC.
- C. Yin, M. Minakshi, D. E. Ralph, Z. Jiang, Z. Xie and H. Guo, J. Alloys Compd., 2011, 509, 9821–9825 CrossRef CAS PubMed.
- Z. Wang, C. Ma, H. Wang, Z. Liu and Z. Hao, J. Alloys Compd., 2013, 552, 486–491 CrossRef CAS PubMed.
- K. Xie, J. Li, Y. Lai, W. Lu, Z. Zhang, Y. Liu, L. Zhou and H. Huang, Electrochem. Commun., 2011, 13, 657–660 Search PubMed.
- M. B. Sassin, A. N. Mansour, K. A. Pettigrew, D. R. Rolison and J. W. Long, ACS Nano, 2010, 4, 4505–4514 CrossRef CAS PubMed.
- W. Qian, F. Sun, Y. Xu, L. Qiu, C. Liu, S. Wang and F. Yan, Energy Environ. Sci., 2014, 7, 379–386 CAS.
- J. P. Paraknowitsch, J. Zhang, D. Su, A. Thomas and M. Antonietti, Adv. Mater., 2010, 22, 87–92 Search PubMed.
- B. Sethuraman, K. Kamaraj Purushothaman and G. Muralidharan, RSC Adv., 2014, 4, 4631–4637 RSC.
- K. S. W. Sing, Pure Appl. Chem., 1982, 54, 2201–22l8 CrossRef.
- V. Ruiz, C. Blanco, M. Granda, R. Menéndez and R. Santamaría, J. Electroanal. Chem., 2008, 618, 17–23 CrossRef CAS PubMed.
- X. J. Jin, M. Y. Zhang, Y. Wu, J. Zhang and J. Mu, Ind. Crops Prod., 2013, 43, 617–622 Search PubMed.
- S. Kondrat, C. R. Perez, V. Presser, Y. Gogotsi and A. A. Kornyshev, Energy Environ. Sci., 2012, 5, 6474–6479 CAS.
- Z. Wang, C. Ma, H. Wang, Z. Liu and Z. Hao, J. Alloys Compd., 2013, 552, 486–491 Search PubMed.
- M. Biswal, A. Banerjee, M. Deoab and S. Ogale, Energy Environ. Sci., 2013, 6, 1249–1259 CAS.
- X. Zhao, C. Johnston and P. S. Grant, J. Mater. Chem., 2009, 19, 8755–8760 RSC.
- X. Xia, Q. Hao, W. Lei, W. Wang, D. Suna and X. Wang, J. Mater. Chem., 2012, 22, 16844–16850 RSC.
- T. Morishitaa, Y. Sonedab, H. Hatorib and M. Inagakia, Electrochim. Acta, 2007, 52, 2478–2484 CrossRef PubMed.
- Y. Wang and Y. Xia, J. Electrochem. Soc., 2006, 153, A450–A454 CrossRef CAS PubMed.
- M. Wu, R. Lee, J. Jow, W. Yang, C. Hsieh and B. Weng, Electrochem. Solid-State Lett., 2009, 12, A1–A4 Search PubMed.
- M. Wu and R. Lee, J. Electrochem. Soc., 2009, 156, A737–A743 CrossRef CAS PubMed.
- X. X. Wang, J. Wang, H. Chang and Y. Zhang, Adv. Funct. Mater., 2007, 17, 3613–3618 Search PubMed.
- M. V. Reddy, T. Yu, C.-H. Sow, Z. X. Shen, C. T. Lim, G. V. S. Rao and B. V. R. Chowdari, Adv. Mater., 2007, 17, 2792–2799 CAS.
| This journal is © The Royal Society of Chemistry 2014 |