A new oil/water interfacial assembly of sulphonated graphene into ultrathin films
Abstract
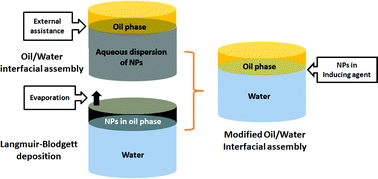
A new oil/water interfacial assembling approach has been developed to fabricate ultrathin graphene films based on a combination of two established interfacial assembling techniques. This new approach integrates multiple advantages such as simple equipment requirements in traditional oil/water interfacial assembly and minimum demand on the feeding source in air/water interfacial assembly (Langmuir–Blodgett deposition). By simply injecting a very small amount (1 ml) of ethanol dispersion of sulphonated graphene sheets (GSs) to the hexane/water interface, GSs can be effectively confined at the interface and self-organize into monolayer film with high uniformity and a controllable topographical feature. Multilayered films can also be obtained by layer-by-layer deposition without extra binding agent, demonstrating a cost-efficient, convenient, flexible and controllable approach for high quality ultrathin graphene films.

 Please wait while we load your content...
Please wait while we load your content...