SERS-based immunoassay of anti-cyclic citrullinated peptide for early diagnosis of rheumatoid arthritis†
Hyangah Chona,
Sangyeop Leea,
Rui Wanga,
So-Young Bangb,
Hye-Soon Leeb,
Sang-Cheol Baeb,
Hyoban Leec,
Bongsoo Kim*c and
Jaebum Choo*a
aDepartment of Bionano Technology, Hanyang University, Ansan 426-791, South Korea. E-mail: jbchoo@hanyang.ac.kr; Fax: +82-31-436-8188
bDepartment of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, 133-792, South Korea
cDepartment of Chemistry, KAIST, Daejeon, 305-701, South Korea. E-mail: bongsoo@kaist.ac.kr; Fax: +82-42-350-2810
First published on 16th July 2014
Abstract
We report a highly sensitive detection method for anti-CCP autoantibodies using a SERS-based magnetic immunosensor. The proposed immunoassay technique is expected to be a new clinical tool for the early diagnosis of rheumatoid arthritis (RA).
Rheumatoid arthritis (RA) is a system autoimmune disease characterized by chronic inflammation of the synovial joints. RA is not curable but it is easily controlled in many patients.1 The earlier the diagnosis is made and treatment begins, the more effectively its progression can be slowed or even stopped.2,3 The goal for RA treatment today is early diagnosis and earliest possible initiation of effective treatment. However, initiation of treatment without a confirmed diagnosis of RA is undesirable because therapies are sometimes toxic and costly. To expedite the diagnostic process, various serum biomarkers have been assessed for improved diagnosis and prognosis of RA. Until recently, autoantibodies against cyclic citrullinated peptide (anti-CCP) in patient serum have been regarded as the best biomarker for early diagnosis of RA.4,5 The advantage of anti-CCP antibodies is that they are detectable in patient serum up to 10 years prior to the appearance of symptoms and, as a diagnostic tool, they have a specificity of 90–95% in patients affected by RA, making them a crucial part of effective diagnosis and treatment of RA.
The most widely used screening tool for the diagnosis of RA is anti-CCP enzyme-linked immunosorbent assay (ELISA). Various types of anti-CCP ELISA test kits for quantitative identification of anti-CCP autoantibodies have been developed and commercialized.6 This test is based on the binding of serum anti-CCP autoantibodies to CCP adsorbed on a microplate surface. More recently, a colloidal gold-based lateral-flow immunoassay kit has been commercialized for a point-of-care (POC) diagnosis of RA. In this case, the patient's sample, along with gold particles, move by capillary force across a channel, and the physician or clinical technician determines, with the naked-eye, whether the color change in the channel has passed a concentration threshold that indicates RA.7 In both methods (ELISA kit and lateral-flow kit), however, sensitivity, specificity, and reproducibility have been a key issue, and a more sensitive and more accurate immunoassay technique for RA diagnosis is still needed.
Recently, surface-enhanced Raman scattering (SERS)-based immunoassay using functional nano-tags has become a promising alternative for sensitive detection in ELISA.8–10 This technique has attracted significant attention because of its rapid and sensitive detection capability. One of the most popular platforms for the SERS-based immunoassay is the detection of sandwich immunocomplexes immobilized on a solid plate.11,12 However, this tool also has several drawbacks, including many repeated washing steps and along incubation time. Recently, our research group developed a new SERS-based competitive immunoassay protocol using magnetic beads to resolve these problems. This method does not use an immobilization procedure on a solid plate; instead, it uses magnetic beads as supporting materials.13–15 In this case, the detection time is much faster than that of the sandwich immunoassay because only one binding step between antibody and antigen is needed. The competitive assay has greater precision and reproducibility but lower sensitivity and specificity than the sandwich assay. In the case of anti-CCP assay for early diagnosis of RA, the detection sensitivity is more important issue than its detection time. Thus, the sandwich immunoassay format was used in this work. In this system, sandwich immunocomplexes, including magnetic beads, are immobilized on the wall of a microtube using a magnetic bar, and then their SERS signals are measured. This technique overcomes the slow immunoreaction problems caused by the diffusion-limited kinetics on a solid plate since the reaction occurs in solution. Consequently, the assay time is greatly reduced to less than 1 h. Furthermore, it is possible to obtain more reproducible results because the SERS signals are measured for the average nanoparticle ensembles in solution.
In this study, we extended the feasibility of the SERS-based detection method using magnetic beads to perform immunoassays for anti-CCP markers for early diagnosis of RA. In the first part of this work, a SERS-based immunoassay for rabbit anti-CCP was evaluated for validation. For this, we prepared CCP-conjugated magnetic beads and antibody-conjugated SERS nano-tags, and the SERS signals were measured for their immunocomplexes. In the second part of this work, we applied the SERS-based assay to human anti-CCP standard solutions purchased as part of a commercially available anti-CCP ELISA kit (BlueGene Biotech, Shanghai, China). The assay results from part one were compared to those obtained from the BlueGene anti-CCP kit in part two to validate its clinical application. To the best of our knowledge, this is the first report of a SERS-based anti-CCP immunoassay, and this approach provides a new direction for early diagnosis of RA.
Fig. 1 illustrates the SERS-based immunoassay process for quantification of anti-CCP autoantibody markers. First, biotinylated CCPs were immobilized on the surface of a streptavidin-bound magnetic bead through a biotin–streptavidin interaction. To find the optimum concentration of CCP, five different concentrations of biotinylated CCP in the 40–400 nM range were reacted with 100 ng mL−1 of anti-CCP and secondary antibody conjugated SERS nano-tags (Fig. S1 in ESI†). Based on the SERS measurements, the optimum concentration of CCP was determined to be 300 nM. Second, different concentrations of anti-CCP autoantibodies were added to bind to and capture CCPs. Here, rheumatoid factor (RF) and bovine serum albumin (BSA) were used as negative controls to evaluate the selective binding capability of anti-CCP autoantibodies. Third, after anti-CCP autoantibodies were selectively captured on the surface of the magnetic beads, their sandwich immunocomplexes were formed by adding secondary antibody-conjugated SERS nano-tags. For this, hollow gold nanospheres (HGNs) were used as SERS nano-tags for highly homogeneous immunoassays of anti-CCP autoantibodies. HGNs are known to have a reproducible enhancement effect on individual particles because of their ability to localize electromagnetic fields through the pinholes of the hollow particle structures.16–18 They can therefore be used as highly reproducible sensing probes for quantitative analysis of immune-specific biomarkers. Here, malachite green isothiocyanate (MGITC) was used as a Raman reporter molecule. Fig. S2 (ESI†) illustrates the sequential procedure for the fabrication of secondary antibody-conjugated SERS nano-tags, and their characterizations using TEM, dynamic light scattering, and UV/Vis absorption spectra. Finally, the anti-CCP sandwich immunocomplexes were isolated through attraction to a magnetic bar on the wall of a microtube, and the residual solution was washed twice with buffer using a micropipette. The magnetic bar was then removed and the immunocomplexes were re-dispersed for SERS detection.
To evaluate the selectivity of our SERS-based immunoassay system, the SERS spectra were measured and analyzed for the same concentration (10 ng mL−1) of rabbit anti-CCP, RF, BSA and their mixtures. As expected, highly enhanced Raman signals were only observed for anti-CCP autoantibody and its mixtures (Fig. 2a). In contrast, no obvious Raman signals were observed for RF or BSA. The relative Raman intensities at 1617 cm−1 in Fig. 2b indicate that the CCP-immobilized magnetic bead only captures the anti-CCP autoantibody, and this SERS-based assay can be used for its selective quantification.
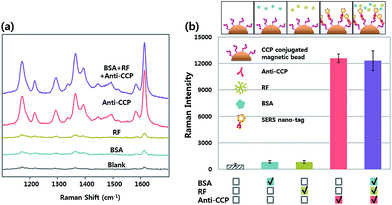 | ||
| Fig. 2 (a) SERS spectra for blank, BSA, RF, anti-CCP and their mixtures and (b) their corresponding SERS intensity at 1617 cm−1. | ||
Fig. S3 (ESI†) shows the TEM images of sandwich immunocomplexes from the 10–400 ng mL−1 range of anti-CCP. When anti-CCPs are present, SERS nano-tags are bound on the surface of magnetic beads by the interaction between anti-CCP and secondary anti-IgG on the HGN surface. The more anti-CCPs present, the more nano-tags will bind on the surface of the magnetic beads. These TEM images provide strong evidence for the increased formation of sandwich immunocomplexes as anti-CCP concentration increases.
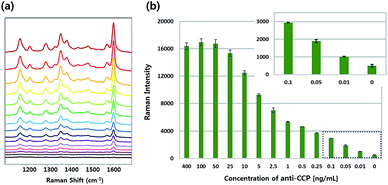
Fig. 3 displays (a) SERS spectra and (b) relative Raman intensities at 1617 cm−1 for various concentrations of rabbit anti-CCP. The SERS spectra were measured for the same concentration range of anti-CCP as the TEM measurements. Here, the characteristic SERS peak intensity at 1617 cm−1 increased concomitantly along with concentration of rabbit anti-CCP antibody (Fig. 3a). When its concentration reached 50 ng mL−1, the formation of immunocomplexes (HGN, SERS, nano-tag-anti-CCP, autoantibody-CCP) was almost complete, as shown in Fig. 3b. It is particularly noteworthy that a consistent SERS intensity change was achieved at the low concentration range (0–100 pg mL−1), as shown in the insert of Fig. 3b. This means that highly accurate quantification of anti-CCP antibody is possible using this SERS-based immunoassay technique. The limit of detection (LOD) for rabbit anti-CCP was estimated to be 13 pg mL−1, based on three standard deviations from the background.
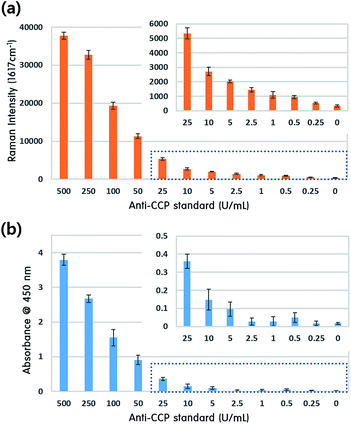
To assess the potential clinical applications of our proposed SERS-based assay method, we compared it to a commercially available anti-CCP ELISA assay kit (BlueGene Biotech). For this comparison, twelve different concentrations of human anti-CCP solutions were prepared. For higher concentration anti-CCP solutions, including 0, 25, 50, 100, 250 and 500 U mL−1, the standard solutions in the kit were used. Anti-CCP solutions lower than 25 U mL−1 were prepared by diluting the standard solution with phosphate buffered saline (PBS). We ran ELISA assays on all these concentrations using the BlueGene kit and protocol. Fig. 4 compares the anti-CCP assay results using our SERS-based immunosensor with those obtained by the BlueGene ELISA assay kit. In the SERS-based assay, the Raman peak intensity at 1617 cm−1 was monitored for its quantitative evaluation.
Overall, the values generated by our proposed SERS assay method (Fig. 4a) show good agreement with those from the ELISA assay kit (Fig. 4b) for various concentrations of standard human anti-CCP solution. Additionally, our SERS-based assay results are more consistent in the low concentration range (0–2.5 U mL−1) than those achieved by the commercial ELISA kit, as shown in the inserts of Fig. 4a and b. This means that more sensitive quantification for human anti-CCP antibody is possible using this SERS-based immunoassay technique. Consequently, it has strong potential to be a new diagnostic tool for early diagnosis of RA.
Conclusions
We developed a new SERS-based immunoassay technique for anti-CCP autoantibody for early diagnosis of RA. Here, CCP-immobilized magnetic beads and secondary anti-IgG conjugated HGNs were used as substrates and SERS nano-tags, respectively. For rabbit anti-CCP autoantibody, the LOD was estimated to be 13 pg mL−1. We also demonstrated that our proposed SERS-based immunosensor is much more sensitive than the commercially available ELISA kit in the low concentration range of human anti-CCP. These assay results demonstrate that this technique has potential value as a new clinical tool for early RA diagnosis. In fact, investigations are currently underway using clinical samples from early RA patients and simultaneous detection of dual RA markers (anti-CCP and RF) to improve the diagnostic sensitivity of the assay.Acknowledgements
This study was supported by a grant from the Korean Health Technology R&D Project, through the Ministry of Health & Welfare, Republic of Korea (grant number: A121983).Notes and references
- L. A. Trouw and M. Mahler, Autoimmun. Rev., 2012, 12, 318–322 CrossRef CAS PubMed.
- W. J. van Venrooij, J. J. B. C. van Beers and G. J. M. Pruijn, Nat. Rev. Rheumatol., 2011, 7, 391–398 CrossRef CAS PubMed.
- D. L. Scott, F. Wolfe and T. W. Huizinga, Lancet, 2010, 376, 1094–1108 CrossRef.
- G. J. M. Pruijn, A. Wiik and W. J. van Venrooij, Arthritis Res. Ther., 2010, 12, 203–204 Search PubMed.
- S. Nijenhuis, A. J. W. Zendman, E. R. Vossenaar, G. J. M. Pruijn and W. J. van Venrooij, Clin. Chim. Acta, 2004, 350, 17–34 CrossRef CAS PubMed.
- X. Bossuyt, D. Coenen, S. Fieuws, P. Verschueren, R. Westhovens and N. Blanckaert, Ann. Rheum. Dis., 2009, 68, 287–289 CrossRef CAS PubMed.
- T. D. Jaskowski, H. R. Hill, K. L. Russo, G. Lakos, Z. Szekanecz and M. Teodorescu, J. Rheumatol., 2010, 37, 1582–1588 CrossRef CAS PubMed.
- C. I. Brady, N. H. Mack, L. O. Brown and S. K. Doorn, Anal. Chem., 2009, 81, 7181–7188 CrossRef CAS PubMed.
- X. X. Han, L. J. Cai, J. Guo, C. X. Wang, W. D. Ruan, W. Y. Han, W. Q. Xu, B. Zhao and Y. Ozaki, Anal. Chem., 2008, 80, 3020–3024 CrossRef CAS PubMed.
- H. Chon, S. Lee, S. W. Son, C. H. Oh and J. Choo, Anal. Chem., 2009, 81, 3029–3034 CrossRef CAS PubMed.
- M. D. Porter, R. J. Lipert, L. M. Siperko, G. Wang and R. Narayanan, Chem. Soc. Rev., 2008, 37, 1001–1011 RSC.
- M. Lee, S. Lee, J. Lee, H. Lim, G. H. Seong, E. Ki. Lee, S.-I. Chang, C. H. Oh and J. Choo, Biosens. Bioelectron., 2011, 26, 2135–2141 CrossRef CAS PubMed.
- H. Chon, S. Lee, S. Y. Yoon, S.-I. Chang, D. W. Lim and J. Choo, Chem. Commun., 2011, 47, 12515–12517 RSC.
- H. Chon, S. Lee, S. Y. Yoon, E. K. Lee, S.-I. Chang and J. Choo, Chem. Commun., 2014, 50, 1058–1060 RSC.
- Y. Yoon, N. Choi, J. Ko, K. Kim, S. Lee and J. Choo, Biosens. Bioelectron., 2013, 42, 62–67 CrossRef PubMed.
- H. Wang, G. P. Goodrich, F. Tam, C. Oubre, P. Nordlander and N. J. Halas, J. Phys. Chem. B, 2005, 109, 11083–11087 CrossRef CAS PubMed.
- A. M. Schwartzberg, T. Y. Oshiro, J. Z. Zhang, T. Huser and C. E. Talley, Anal. Chem., 2006, 78, 4732 CrossRef CAS PubMed.
- C. E. Talley, J. B. Jackson, C. Oubre, N. K. Grady, C. W. Hollars, S. M. Lane, T. Huser and N. J. Halas, Nano Lett., 2005, 5, 1569–1574 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, TEM images, dynamic light scattering, and UV/Vis absorption spectral data. See DOI: 10.1039/c4ra05149a |
| This journal is © The Royal Society of Chemistry 2014 |