α-MoO3 nanoparticles: solution combustion synthesis, photocatalytic and electrochemical properties†
Abstract
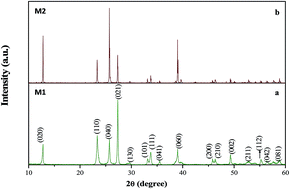
Nanoparticles of ultra-porous MoO3 were synthesized in a single step by a solution combustion reaction using molybdenum metal powder as precursor for the first time. The effects of the preparation conditions, such as the temperature and precursor concentration, on the crystalline phase and morphology of the products were studied systematically. The analytical techniques SEM, TEM, PXRD, TGA, FTIR, and SAED were used to characterize the morphology, composition, and structure of the as-prepared products. The TEM images of MoO3 show the sizes of the particles to be in the range of 2–10 nm. Electrochemical characterization of MoO3 was carried out in 0.5 M H2SO4. The specific capacitance and electrochromic properties of MoO3 were studied. The high photocatalytic activity of MoO3 was investigated using methylene blue azo dye at various concentrations. MoO3 showed the ability to degrade 100% of methylene blue present at high concentrations of about 75 mg L−1.

 Please wait while we load your content...
Please wait while we load your content...