An aggregation-induced emission-based fluorescent chemosensor of aluminium ions†
Abstract
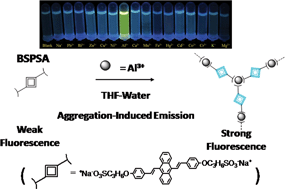
Here we used an aggregation-induced emission (AIE) molecule, 9,10-bis[4-(3-sulfonatopropoxyl)-styryl]anthracene sodium salt (BSPSA), to act as a highly selective and sensitive fluorescent chemosensor for the detection of aluminium ions. This would widen the applications of AIE luminogens, and may show potentials for aluminium ion detection.

 Please wait while we load your content...
Please wait while we load your content...