A high performance xylose microbial fuel cell enabled by Ochrobactrum sp. 575 cells†
Xin Liabc,
Guo-Zhen Zhongabc,
Yan Qiao*abc,
Jing Huangabc,
Wei Hua Huabc,
Xing-Guo Wangd and
Chang Ming Li*abc
aInstitute for Clean energy & Advanced Materials, Faculty of Materials & Energy, Southwest University, Chongqing 400715, China. E-mail: ecmli@swu.edu.cn; yanqiao@swu.edu.cn
bChongqing Key Laboratory for Advanced Materials and Technologies of Clean Energies, Chongqing 400715, China
cChongqing Engineering Research Center for Rapid diagnosis of Fatal Diseases, Chongqing 400715, China
dFaculty of Life Sciences, Hubei University, Wuhan 430062, China
First published on 12th August 2014
Abstract
A new strain, Ochrobactrum sp. 575, is applied as an anodic biocatalyst in a xylose MFC. After evolution under electrochemical tension in MFCs, Ochrobactrum sp. 575 can deliver a maximum power density of 2625 mW m−3, which is 20 times higher than that reported for similar MFCs. The slight increase in pH with the operation progress of Ochrobactrum sp. 575 MFC indicates proton involvement in the anode electrochemical reaction. For the first time, fumaric acid, an important intermediate in the succinate oxidation respiratory chain of gram-negative strains is discovered in the anodic supernatant of the MFC. This indicates that the process of xylose digestion with Ochrobactrum sp. 575 depends on the succinate oxidation respiratory chain, which is quite different from the traditional NADH oxidation respiratory chain in other electroactive bacterial strains. The significant improvement in the MFC power density is likely to be attributed to fumaric acid generated by bacteria cells as an electron mediator to facilitate electron transfer during the discharging process.
Introduction
Microbial fuel cells (MFCs) are devices that use microbes to convert the chemical energy stored in organic compounds and organic wastes into electricity.1 It has been more than 100 years since the first report on bacterium-produced electricity2 but only in recent years have MFCs been developed as a promising green energy source. MFCs possess a number of prominent merits like degrading organic matter, mild operation conditions and low cost.3,4 MFCs also are very promising for simultaneously enabling waste treatment and electricity generation.3,5,6 A wide range of soluble or dissolved complex organic wastes and renewable biomass can be used as MFC fuels. The major substrates can be various kinds of artificial and real wastewater as well as lignocellulosic biomass.7 In recent years, the production of fuel and energy from lignocellulosic biomass, such as agricultural residues and woody biomass have drawn great attention because of the abundance, ready availability and renewable nature.8,9As the most representative pentose, xylose is one of the main compositions of lignocellulosic hydrolysates, which comprises up to 13–23% of the organic matter in aqueous hydrolysates from lignocellulosic biomass.10,11 The utilization of xylose in MFCs provides a new approach for the generation of renewable energy from biomass. To date, most of the xylose MFCs utilize a mixed bacteria culture as a biocatalyst.1,12,13 In such a complicated system, it is hard to understand the detailed mechanism of the energy conversion and electron transfer. In this case, there is no clue to improve the cell design for enhancing the performance. An obvious solution is to choose a single strain with high catalytic activity as the catalyst for xylose MFCs.
In this work, we used a new strain, Ochrobactrum sp. 575, which can use xylose as the sole carbon source in MFCs. The power generation performance and electrocatalytic activity of this strain were investigated. To understand the direct electron transfer process in the xylose MFC, electrochemical characterization combined with spectrum analysis of the supernatant were investigated. A succinate oxidation respiratory chain involved mechanism is proposed to explain the phenomenon.
Experimental
MFC construction
The dual-chamber MFC used in this work was constructed with two bottles (100 mL capacity) separated by a proton exchange membrane. The chambers were sealed by rubber stoppers that punched a hole in the middle to allow a titanium wire to pass through. The proton exchange membrane was boiled sequentially in 0.5% H2O2, deionized water, 1% H2SO4, and deionized water for 5 min each. The carbon fiber brush electrodes (10 mm diameter × 20 mm length) made from carbon fibers and titanium wire14 were used as the anode and cathode after cleaning with deionized water, followed by drying at 100 °C and UV-sterilizing for 3 h in a biological safety cabinet. The anolyte was the xylose medium and the catholyte was 50 mM K3[Fe(CN)6] in 0.1 M PBS. An external load (1.8 kΩ) was used to evaluate the long term discharge performance. The polarization and power output curves were measured by varying the output load resistor from 0.2 kΩ to 8 kΩ to monitor the steady-state current.Bacterial cultivation
Ochrobactrum sp. 575 (preserved by China Center for Type Culture Collection, CCTCC M2013549) was isolated from root zoon soil and has been proved using xylose as the sole carbon source. The bacteria cells from a single colony on a lysogeny broth (LB) agar plate was inoculated in 5 mL LB medium for overnight culturing at 37 °C, 180 rpm. The overnight culture was then inoculated in the xylose medium, which contained 1 g NaHCO3, 0.85 g NaH2PO4, 0.5 g yeast extract, and 1 g xylose per 100 mL.15 After the cell culture reached a steady state (OD600 = 1.2), the bacteria cells were harvested by centrifugation at 4 °C, 6000 rpm, 5 min, resuspended in xylose medium, and then transferred to the anodic chamber of the MFC. After the output voltage dropped down, the bacteria cells in anolyte were inoculated on fresh LB agar plate to obtain the single clone of the evolved bacteria cells. All the chambers, rubber stoppers and media were autoclaved at 121 °C and the xylose solution was filtered through a 0.22 μm membrane to remove any microorganisms. Before the test, the suspension was purged with nitrogen for 30 min to remove oxygen.Observation of biofilm anodes
Scanning electron microscopy (SEM, JSM-6510LV, Japan) was used to examine the morphology of the bacteria adhered anodes. For sample preparation, a piece of carbon brush was cut from the anode after discharge and immersed in 2.5% glutaraldehyde in PBS buffer for 2 hours to fix the morphology of the bacteria cells. After a series dehydration with 30%, 40%, 50%, 60%, 70%, 80%, 90%, and 100% ethanol (20 min each rinse), the samples were dried in a vacuum oven at room temperature for the SEM observations.16Electrochemical characterization
Electrochemical experiments were conducted in a three-electrode cell with carbon brushes as the working and counter electrodes and Ag/AgCl (saturated KCl) as the reference electrode. A potentiostat (CHI660E, Shanghai Chenhua, China) was used for all cyclic voltammetry (CV) measurements that were conducted at scan rate of 30 mV s−1 or 1 mV s−1 with the potential range from −0.8 V to −0.1 V.17Spectrum analysis of anolyte supernatant
After two discharge cycles, the anolyte was centrifuged and stored at 4 °C prior to analysis. Fourier transform infrared (FT-IR) spectra of the supernatant were examined ranging from 4000 cm−1 to 400 cm−1 in transmission mode. The UV-Vis spectra were measured from 200 nm to 700 nm using a quartz cell with a 1 cm path length. The pH of the anodic chamber was measured using a pH meter. All the tests were conducted at room temperature.The supernatant of the anolyte was also subjected to HPLC-MS measurements. Preparative HPLC was performed using ELITE P-230 pumps, and the optical rotations were measured on a JASCO P-1030 digital polarimeter. High-resolution (HR) MS measurements were performed on an Apex III (7.0 Tesla) FT-ICR mass spectrometer (Bruker, Billerica, MA, USA) using CF3COONa as an external calibration standard.
Results and discussion
Power generation of Ochrobactrum sp. 575 MFC
When the original Ochrobactrum sp. 575 was applied as an anodic catalyst for the MFC, the open circuit voltage was only 180 mV and the power density was about 85 mW m−3 at a constant load of 1.8 k ohms (Table S1†). To enhance the electrocatalytic performance of the strain, the cell cultures were inoculated from the anode chamber after a long term discharge (about 140 hours) and the obtained single clone (noted as Generation I) was inoculated in the anode of another MFC. The MFC catalyzed by Generation I cells delivered an open circuit voltage of 330 mV and a power density of 205 mW m−3. The same treatment was repeated two times to obtain the Generation II and Generation III cells. Finally, the Generation III cells catalyzed MFCs achieved an open circuit voltage of 680 mV and a power density of 1473 mW m−3, which is around 17 times higher than the original cells. To explain the reason for the greatly improved performance of the Generation III cells, the morphology of the anodes was observed after 140 h of discharge. Fig. 1 shows that for the original bacteria catalyzed anode, only a few bacteria cells adhered to the carbon fiber surface (Fig. 1a and b). While for the Generation III cells catalyzed anode, a high density of cells attached to the carbon fiber and formed a biofilm (Fig. 1c and d). This phenomenon suggests that after evolution, the cells are willing to adhere to the anode surface, which will facilitate interfacial electron transfer between the cells and the anode.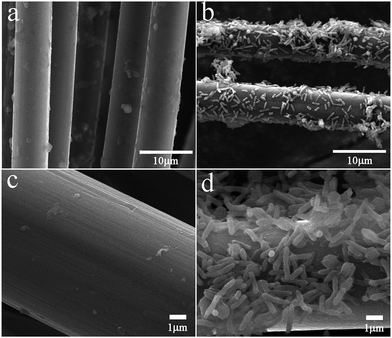 | ||
| Fig. 1 SEM images of the original Ochrobactrum sp. 575 (a and b) and Generation III Ochrobactrum sp. 575 cells (c and d) adhered carbon fiber anodes. | ||
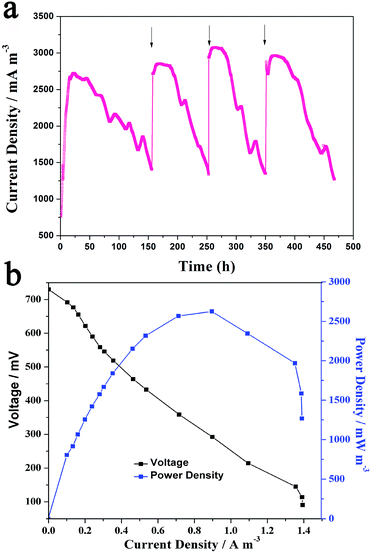
The xylose MFC catalyzed by Generation III cells were running at room temperature for four cycles and the current generation profile was recorded (Fig. 2a). In the first 20 h of the first cycle, the current increased slowly and reached the maximum value (around 2700 mA m−3) at about 24 h, which was maintained for the next 24 h, and then began to decrease slowly. When the current decreased to half of the maximum value, the anolyte was replaced with fresh medium to start the next cycle. It is noted that there was no lag time for the increase in current in the following three cycles. The voltage reached the maximum plateau in several hours. The reason might be that the biofilm form on the anode could undergo the fast oxidation of xylose and pass the electrons to the anode. The four cycles of voltage output indicated the MFC could discharge steadily and repeatedly for a long time. Fig. 2b shows the polarization curve and power curve of the MFC. The maximum power density reached 2625 mW m−3 at a current density of 8985 mA m−3, which is 22.2 times higher than the reported xylose MFC.13 Since the Generation III cells could deliver greater performance in the xylose MFCs, they were used for the following electrochemical and spectral analysis.
Electrocatalytic behavior of evolved Ochrobactrum sp. 575 anode
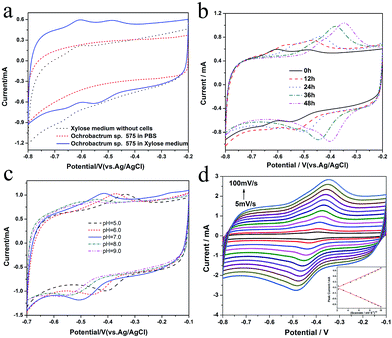
In order to explore the electrocatalytic mechanism of Ochrobactrum sp. 575, its electrochemical behavior in the MFC anode was investigated. Fig. 3a shows that two pairs of redox peaks can be observed on the cyclic voltammogram of Ochrobactrum sp. 575 cell cultures with the xylose medium. However, when the cells were suspended in phosphate buffer, no peak could be found, which indicates that the redox peaks could be due to the redox reaction of certain xylose metabolic products. The CV curve (Fig. S1†) obtained at a low scan rate (1 mV s−1) clearly shows the catalytic current and the onset potential of xylose oxidation of ∼−0.38 V. With increasing discharging time, the redox peaks shifted to positive potential and the peak current of the more positive redox pair increased significantly (Fig. 3b), which could be due to new metabolic products generated or the redox potential changed from the pH variation during discharging. To find an explanation, the cyclic voltammograms of the anolyte at different pH were measured (Fig. 3c). The results show that when the pH is 9, two pairs of redox peaks can be found and they shift to a positive potential when the pH value decreases to 8. While when the pH decreases to 7, the more positive redox pair increases a lot but the more negative peaks decreases. Only one pair of peaks can be observed when the pH is lower than 7 and this redox pair shifts to positive with decreasing pH. These results suggest that the proton is involved in the redox reaction of the anode. Furthermore, the relationship between the scan rate and the peak current was investigated at pH 7. The redox peak current showed a linear relationship with the square root of the scan rate (Fig. 3d), revealing a diffusion control process. According to an analysis of the electrochemical behavior, it could be concluded that the Ochrobactrum sp. 575 cells digest xylose and generate some kind of metabolite that possesses redox activity. The redox reaction of this metabolite includes a dehydrogenation/hydrogenation process.Analysis of the supernatant of the anolyte
The pH of the anolyte was measured during a cycle of MFC discharge (Fig. 4). The pH declined greatly along with the rapid increasing cell voltage in the first 20 h, and the pH decreased slowly when the voltage of the MFC was stable and decreased. This suggests that a large amount of organic acids are generated during the start up period until the voltage reaches the maximum, and is in agreement with the electrocatalytic analysis results.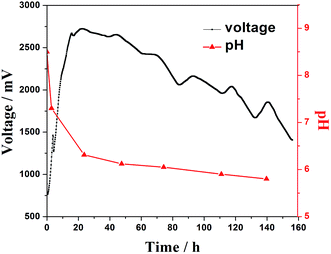 | ||
| Fig. 4 Variation of the pH value in the anodic chamber and MFC output voltage in a cycle of discharge. | ||
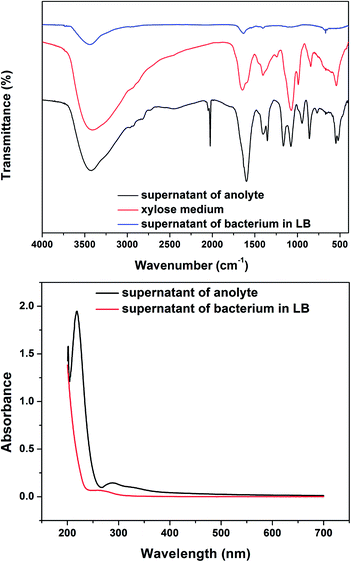
To clarify the organic acid generated from the xylose metabolism, the FTIR and UV-Vis spectra of the anolyte supernatant, xylose medium and the supernatant of bacteria culture in the LB medium were examined. The FTIR spectra of the supernatant presented a strong and poignant peak at 2025 cm−1, which does not appear in the xylose medium (Fig. 5a). The peak indicated olefin or alkyne existing in the supernatant that was produced by the metabolism of bacteria cells. In Fig. 5b, the supernatant of anolyte displays a strong absorption at 220 nm in the UV-Vis spectrum, indicating the conjugated structure. Since the supernatant of bacteria cultured in LB does not have such profiles in the FTIR spectra and UV-Vis absorption, it suggests that the Ochrobactrum sp. 575 cells do not generate such organic acids when fed with LB medium. Boily et al. reported that fumaric acid had a peak absorption at 220 nm in the UV-Vis spectra.18 According to the analysis above, we propose that the organic acid in the supernatant that possesses an olefin structure could be fumaric acid. For further verification, the supernatant was analyzed by HPLC-MS. As shown in Fig. S2,† the peak at m/z of 173.37 may indicate the presence of fumaric acid in the suppernant. In addition, the cyclic voltammograms of fumaric acid in xylose medium is similar to supernatant of anolyte (Fig. S3†). When the anolyte was replaced with the fresh xylose medium in the MFC, the discharging current dropped significantly (Fig. S4†). The addition of fumaric acid in (1 mg mL−1) resulted in a fast increase in the discharge current as well as the current plateau. The results indicate that fumaric acid plays an important role in the catalytic process of this xylose MFC.
Mechanism for high power generation performance of Ochrobactrum sp. 575 MFC
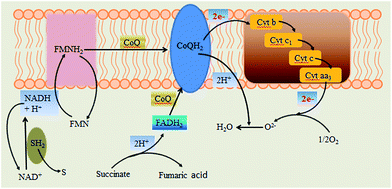
According to the results and analysis, the high power output of Generation III Ochrobactrum sp. 575 MFC should be attributed to the fast digestion of xylose by the cells and the fumaric acid produced from the evolved cells for rapid extracellular electron transfer. The accumulation of fumaric acid in the MFC anode suggests a succinate-involved respiratory chain of Ochrobactrum sp. 575 cells. In microorganisms, there are two types of respiratory chains. One involves NADH oxidation, which is found in most microorganisms, and the other includes succinate oxidation (Fig. 6), of which the main difference is due to their different electron donors, NADH or succinate. When succinate is used as the electron donor, fumaric acid is generated.19,20 It has been proven that fumaric acid is the metabolic intermediate of many strict anaerobes and facultative anaerobes.21 The observed fumaric acid in the xylose MFC anode indicates that the succinic acid rather than NADH could be an important electron donor for electron transport. The relationship between the electrocatalytic activity of the bacterial strain and the change in its electron transport profile is still under investigation.Conclusions
In this work, the electrocatalytic behavior of Ochrobactrum sp. 575 for MFC anode was investigated and a power density of 2625 mW m−3 was achieved. This is a significant improvement for a single strain catalyzed MFC compared to the reported mixed culture catalyzed MFCs. The accumulation of fumaric acid in the anodic chamber was observed during the progress of xylose oxidation by Ochrobactrum sp. 575 cells. This work provides a new bacteria strain for MFCs to harvest electricity while resolving the xylose, and offers a new strain for fundamental studies of electron transfer-involved mechanisms in a bioelectrocatalytic process.Acknowledgements
We thank Professor Xingguo Wang, Hubei University, for kindly providing the bacterial strain Ochrobactrum sp. 575. This work was financially supported by the Chongqing Key Laboratory for Advanced Materials and Technologies of Clean Energies, the Start-up grant under SWU111071 from Southwest University, the Chongqing Science and Technology Commission under cstc2012gjhz90002, Chongqing, China, the Fundamental Research Funds for the Central Universities (XDJK2013C022), and the National Natural Science Foundation of China (no. 31200102).Notes and references
- L. Huang and B. E. Logan, Appl. Microbiol. Biotechnol., 2008, 80, 655–664 CrossRef CAS PubMed.
- M. C. Potter, Proc. R. Soc. London, Ser. B, 1911, 84, 260–276 CrossRef.
- Y. Qiao, S.-J. Bao and C. M. Li, Energy Environ. Sci., 2010, 3, 544 CAS.
- J. Liu, Y. Qiao, Z. S. Lu, H. Song and C. M. Li, Electrochem. Commun., 2012, 15, 50–53 CrossRef CAS PubMed.
- D. R. Lovley, Nat. Rev. Microbiol., 2006, 4, 497–508 CrossRef CAS PubMed.
- B. E. Logan, Nat. Rev. Microbiol., 2009, 7, 375–381 CrossRef CAS PubMed.
- D. Pant, G. Van Bogaert, L. Diels and K. Vanbroekhoven, Bioresour. Technol., 2010, 101, 1533–1543 CrossRef CAS PubMed.
- L. Petrus and M. A. Noordermeer, Green Chem., 2006, 8, 861–867 RSC.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS PubMed.
- A. B. Thomsen, A. Thygesen, V. Bohn, K. V. Nielsen, B. Pallesen and M. S. Jørgensen, Ind. Crops Prod., 2006, 24, 113–118 CrossRef CAS PubMed.
- B. Palmarola-Adrados, P. Chotěborská, M. Galbe and G. Zacchi, Bioresour. Technol., 2005, 96, 843–850 CrossRef CAS PubMed.
- L. Huang and I. Angelidaki, Biotechnol. Bioeng., 2008, 100, 413–422 CrossRef CAS PubMed.
- L. Huang, R. J. Zeng and I. Angelidaki, Bioresour. Technol., 2007, 99, 4178–4184 CrossRef PubMed.
- B. Logan, S. Cheng, V. Watson and G. Estadt, Environ. Sci. Technol., 2007, 41, 3341–3346 CrossRef CAS.
- Y. Qiao, C. M. Li, S. J. Bao, Z. Lu and Y. Hong, Chem. Commun., 2008, 1290–1292 RSC.
- J. Liu, Y. Qiao, C. X. Guo, S. Lim, H. Song and C. M. Li, Bioresour. Technol., 2012, 114, 275–280 CrossRef CAS PubMed.
- Y. Qiao, C. M. Li, Z. Lu, H. Ling, A. Kang and M. W. Chang, Chem. Commun., 2009, 6183–6185 RSC.
- J.-F. Boily and T. M. Seward, J. Solution Chem., 2005, 34, 1167–1190 CrossRef CAS.
- W. H. Elliott, D. C. Elliott, J. R. Jefferson and J. Wheldrake, Biochemistry and molecular biology, Oxford University Press Oxford, 1997 Search PubMed.
- K. Ahmadi and M. Waterfield, Encyclopedia of Biological Chemistry, Elsevier/Academic Press, 2004 Search PubMed.
- H. Song and S. Y. Lee, Enzyme Microb. Technol., 2006, 39, 352–361 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05077k |
| This journal is © The Royal Society of Chemistry 2014 |