Rheological characterization of mammalian lung mucus†
Erick S. Vasqueza,
Jacquelyn Bowserb,
Cyprianna Swiderskib,
Keisha B. Walters*a and
Santanu Kundu*a
aDave C. Swalm School of Chemical Engineering, Mississippi State University, MS, USA. E-mail: santanukundu@che.msstate.edu; kwalters@che.msstate.edu
bDepartment of Clinical Sciences, College of Veterinary Medicine, Mississippi State University, USA
First published on 30th July 2014
Abstract
Mammalian lung mucus is a complex fluid that demonstrates non-linear viscoelastic responses, strain-stiffening at low and strain-softening at large strain values, as measured using large amplitude oscillatory shear (LAOS) experiments. The mechanical properties of lung mucus reported here can be linked to high-strain rate physiological processes, such as coughing, and can guide drug delivery development.
Mucus is a heterogeneous and gel-like material that protects several organs, such as the respiratory tract, GI-tract, and reproductive tract from bacteria, viruses, toxins, and foreign particles. The major components of mucus are water and mucin, which are o-linked glycoproteins with different monomer/oligomer configurations.1–3 Minor quantities of cellular proteins (albumin, enzymes, and immunoglobulin), DNA, lipids, ions and mineral salts are also present in mucus.2–6 The intrinsic gel-like characteristics of mucus are caused by the several forms of inter- and intra-molecular bonding of mucus constituents.
Mucus is ubiquitous in mammals, however, due to its complex nature, very little is known about the physical characteristics of mucus, particularly, the relationships between mechanical properties, microstructure—including the size, assembly and crosslinking interaction of polymeric mucin chains,7 and physiological functions. Here, we report the mechanical (viscoelastic) properties of horse (mammalian) lung mucus measured by using steady-shear and small-/large-strain oscillatory shear flow. We developed a unique experimental protocol to harvest mammalian mucus and our results elucidate complex rheological behavior of mammalian pulmonary mucus, including a strain-stiffening response not reported earlier. We anticipate that a better understanding of the horse lung mucus mechanical properties, as reported here, will be applicable to other mammals, including humans. These results will lead to the development of more-efficient drug delivery systems and will also provide insight on physiological processes such as coughing and potential changes of these physiological processes as a result of lung diseases.
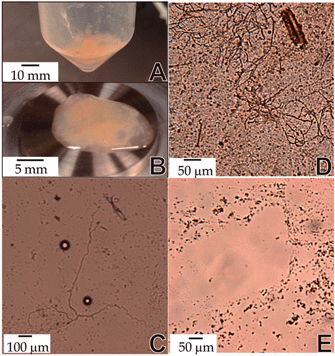
Obtaining mucus samples in sufficient quantities to apply various characterization techniques is a significant challenge. In general, human and/or animal mucus is obtained using bronchoalveolar lavage (BAL) methods. Typically, a fiber optic bronchoscope is inserted into a specific bronchus tube of the lung, followed by a 0.9% NaCl solution instillation, and fluid recovery using suction or syringe methods. However, using this method, only a small amount of mucus can be obtained. Alternatively, to obtain a significant quantity of sample, a lavage method was used in this study on a postmortem horse with a healthy respiratory tract (ESI 1†). Briefly, a saline solution (0.9% NaCl) was infused through an endotracheal tube into the distal trachea and lungs of a postmortem horse. External compression was applied to the thorax before allowing the liquid to egress from the tube by lowering the animal's head. The extracted liquid was subjected to multiple centrifugation steps (1 h, 4180 rcf) until mucus was separated, as shown in Fig. 1A (ESI 1†). The mucus was immediately characterized using rheology (Fig. 1B) and optical microscopy (Fig. 1C–E), or refrigerated (∼ 4 °C) for later analysis.
The morphology of mucus dictates the mechanical properties, and a microstructural analysis at different pH conditions was conducted, as shown in Fig. 1. Interestingly, as-collected mucus, in the presence of physiologic saline solution, displayed mostly fiber-like structures (Fig. 1C). Different aliquots of centrifuged mucus fluid were transferred to HCl solutions (1% v/v). In an acidic pH (∼2) condition, multiple fibers were shown to aggregate as compared with the ‘as-collected’ mucus (Fig. 1D). This behavior has been previously observed for gastric mucin, and is attributed to the hydrophobic and electrostatic interactions between mucins at low pH.8 By tuning the pH (5.5), these aggregates were no longer observed (Fig. 1E); however, a different morphological structure evolved. As lung mucus samples were dried, a branching structure was observed (ESI 2†).
Mucus structure depends on disulfide, electrostatic, and/or hydrophobic interactions, where the macromolecular mucin chains can self-assemble based on the environmental conditions.5 Thus, mammalian lung mucus morphology is very complex and dynamic, and is a strong function of the fluid pH and salt concentrations. The mechanical properties are also affected by hydration level and solids content of mucus. As measured by using thermogravimetric analysis (TGA), the total solid content for the ‘native’ horse lung mucus was 2.16 ± 0.22% (ESI 3†), comparable to the values reported in previous mammalian lung mucus studies.3,5 To examine the connection between external environmental triggers such as commercially available drugs and surface-modified nanoparticles with the dynamic mucus constituents, further studies will characterize the microstructure of mucus using surface sensitive techniques as reported elsewhere.9–12
Several physiological/external factors such as hypersecretion of airway mucus,6 recurrent airway obstruction,13 particle clearance and retention,14 airborne infections including bacteria and pathogens,7 ciliary and cough transport, and subject age5 can play a significant role on the rheological properties of mucus. Thus, it is important to define mucus origin, diseases, or any external factor that could influence the mechanical response of the mucus gel. Gross necropsy findings and histopathological examination of lung sections, optical microscopy, and thermogravimetric analysis of the collected mucus confirmed that lung mucus samples from this horse were suitable for rheological/mechanical characterization.
In realistic physiological scenarios, from ciliary clearance to coughing and sneezing, lung mucus is subjected to a range of shear/strain rates.1 For example, high shear-rate values (∼1000 s−1) are reached during coughing, bronchoconstriction, or during inhaler administrations.5,15 Therefore, a better understanding of lung function, particle clearance, effective drug administration, barrier-functional properties of mucus, and biological systems computational modeling16 can be gained from rheological characterization.
Viscosity as a function of shear rate for three lung mucus samples is shown in Fig. 2 (experimental conditions: ESI 4†). A non-Newtonian shear-thinning behavior was observed for these samples. Similar shear-thinning behavior has been observed for mucus from different organs (e.g. lung, cervical, nasal) from humans and other mammals.5 Only a limited number of studies reported the rheological properties of horse lung mucus; these studies used either dynamic oscillatory shear measurements,9 or apparent viscosity analysis obtained with endoscopic scoring methods.17 As previously stated, during physiological processes such as coughing and sneezing, the shear rates can go as high as 103–104 s−1. Interestingly, at those shear rates mucus displays shear-thinning behavior, which facilitates airway clearance processes.5
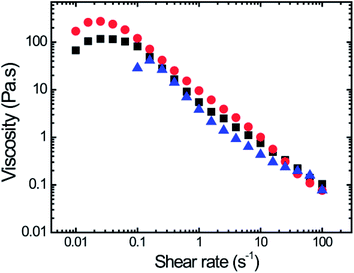 | ||
| Fig. 2 Viscosity vs. shear rate for three different horse mucus samples demonstrating shear thinning behavior. | ||
At low shear rates, the viscosity values reach a plateau and in some instances show a slight shear-thickening behavior. Previous studies have reported the shear-thickening behavior at low shear rate for human respiratory sputum, followed by a shear-thinning response, which depends on mucus points of extraction (trachea or bronchus tube).18 In this study, mucus was extracted from the entire lung. Future studies will be performed on horse lung mucus as a function of mucus extract locations to confirm the rheological behavior observed in these whole-lung mucus samples (Fig. 2).
Steady shear rheological measurements have a limited applicability for crosslinked, gel-like materials, as the inherent network structure can be destroyed at higher strains and strain-rates. Therefore, the mechanical properties of gels are commonly characterized by small amplitude oscillatory shear (SAOS) rheological measurements.11,19 As several forms of physical crosslinking are present in mucus, oscillatory shear experiments were performed to obtain a better understanding of the viscoelastic properties of lung mucus. Fig. 3 displays the storage modulus (G′) and the loss modulus (G′′) as a function of strain for a constant angular frequency (ω = 1 rad s−1) for three replicate lung mucus samples obtained from a single postmortem horse. Here, G′ is higher than G′′ except at very high strain values, indicating a gel-like (soft-solid) behavior. Additionally, G′ is higher than G′′ at different angular frequencies for a constant strain (γ0 = 2%) (ESI 4†). This behavior is likely caused by the physical crosslinking present in mucus samples. The plateau G′ values for the three samples vary from 3–30 Pa, indicative of the heterogeneous nature of mucus.5 Reported values for G′ and G′′ for horse lung mucus, obtained with a magnetic microrheometer, varied between 18–34 Pa, and 6–12 Pa, respectively, the same order of magnitude reported here.5
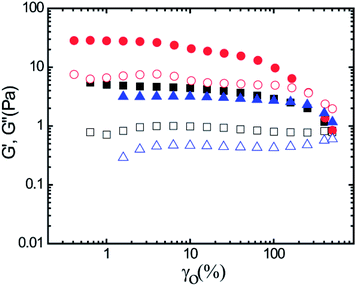 | ||
| Fig. 3 Storage modulus, G′ (filled symbols), and loss modulus, G′′ (open symbols), for three horse lung mucus samples measured at a constant angular frequency (ω) of 1 rad s−1. | ||
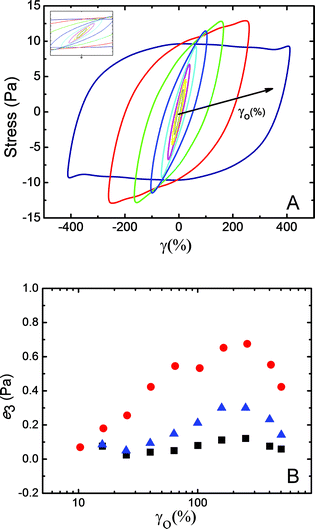
At high-strain, G′ decreases significantly, i.e., mucus shows an apparent strain-softening behavior. A cross-over with G′′ was also observed, indicating disruption of mucus network structure at large-strain (Fig. 3). It was observed that mucus strain-soften at large-strain (or large amplitude oscillatory strain) and as these results lie beyond the linear viscoelastic regime, mathematical framework developed by Ewoldt et al. was utilized to analyze large amplitude oscillatory shear (LAOS) data.20–24
The data analysis was performed using MITLaos software.21,24 Here, symmetry argument is applied to decompose the nonlinear stress response into elastic and viscous stresses. The elastic and viscous stresses for every cycle were then represented with an nth order Chebyshev polynomial of the first kind. The coefficients e1, e3 are obtained for the elastic stress, whereas, ν1, ν3 are obtained for the viscous stress as shown in eqn (1) and (2), respectively.21
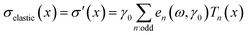 | (1) |
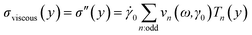 | (2) |
Considering orthogonality, x = γ/γ0 and y = ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/char_e0a2.gif) /
/![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/char_e0a2.gif) 0 are used in a selected domain (−1, +1) and Tn indicates the nth-order Chebyshev polynomial of the first kind. The elastic and viscous Chebyshev coefficients (en and νn, respectively) provide insight about the inter/intra-cycle nonlinearities. At low strain values, i.e., in the linear viscoelastic regime, e1 and ν1 are represented by G′ and η′.
0 are used in a selected domain (−1, +1) and Tn indicates the nth-order Chebyshev polynomial of the first kind. The elastic and viscous Chebyshev coefficients (en and νn, respectively) provide insight about the inter/intra-cycle nonlinearities. At low strain values, i.e., in the linear viscoelastic regime, e1 and ν1 are represented by G′ and η′.
Fig. 4 displays the smoothed stress–strain responses (Lissajous–Bowditch curves) obtained for a representative mucus sample. For small strain values, typical elliptical responses were observed (Fig. 4A, inset). The response curves became distorted at higher-strain values, indicating nonlinear viscoelastic responses. The third elastic Chebyshev coefficient (e3) provides insight about the strain stiffening/softening behavior. As shown in Fig. 4B, with increased strain the values of e3 initially increase and then decrease for all three mucus samples. This indicates that the mucus strain-stiffens slightly before strain-softening. Similarly, ν3 displayed mostly shear thinning behavior. LAOS investigations of mucus samples are rarely reported. However, in a recent study on gastropod pedal mucus, strain-stiffening behavior has also been reported.21,23
Due to the presence of mucin fibers, stiffening behavior is expected as the strain increases in order to protect the lung tissue to a certain level of strain. However, as coughing and sneezing occur, airway secretion and clearance are also facilitated by the mucus layer. Thus, at very large strain/strain-rates when coughing occurs, 103–104 s−1,5 a strain-softening behavior should be expected on mucus in a healthy specimen as shown by LAOS results.
Conclusions
Horse lung mucus is a complex biofluid with nonlinear viscoelastic characteristics as shown by Lissajous–Bowditch curves and LAOS analysis. The strain-stiffening behavior followed by a strain-softening behavior provides new physical understanding for mammalian lung mucus viscoelastic behavior at large strains. These results can be used to model coughing and other high-strain rate physiological processes such as drug delivery mechanisms, particle transport and airway clearance inside the lung, self-assembly and crosslinking of mucus materials, and interactions of mucus with external materials.Acknowledgements
This work was supported by the National Science Foundation [EPS-0903787] and [CBET-0933493]. We are very grateful to Santosh Kumar T. K. and Dr Alba Rodil from College of Veterinary Medicine at Mississippi State University who assisted in mucus collection. Dr Priscilla Hill is also gratefully acknowledged for providing access to the optical microscope used in this study.References
- R. A. Cone, Adv. Drug Delivery Rev., 2009, 61, 75–85 CrossRef CAS PubMed.
- M. King and B. K. Rubin, Adv. Drug Delivery Rev., 2002, 54, 1475–1490 CrossRef CAS.
- R. Bansil and B. S. Turner, Curr. Opin. Colloid Interface Sci., 2006, 11, 164–170 CrossRef CAS PubMed.
- K. Khanvilkar, M. D. Donovan and D. R. Flanagan, Adv. Drug Delivery Rev., 2001, 48, 173–193 CrossRef CAS.
- S. K. Lai, Y. Y. Wang, D. Wirtz and J. Hanes, Adv. Drug Delivery Rev., 2009, 61, 86–100 CrossRef CAS PubMed.
- W. D. Kim, Eur. Respir. J., 1997, 10, 1914–1917 CrossRef CAS.
- D. J. Thornton, K. Rousseau and M. A. McGuckin, Annu. Rev. Physiol., 2008, 70, 459–486 CrossRef CAS PubMed.
- Z. Hong, B. Chasan, R. Bansil, B. S. Turner, K. R. Bhaskar and N. H. Afdhal, Biomacromolecules, 2005, 6, 3458–3466 CrossRef CAS PubMed.
- S. V. Orski, S. Kundu, R. Gross and K. L. Beers, Biomacromolecules, 2013, 14, 377–386 CrossRef CAS PubMed.
- E. Eyiler, I. W. Chu and K. B. Walters, J. Appl. Polym. Sci., 2014, 40888 Search PubMed.
- M. S. Waters, S. Kundu, N. J. Lin and S. Lin-Gibson, ACS Appl. Mater. Interfaces, 2013, 6, 327–332 Search PubMed.
- E. S. Vasquez, I. W. Chu and K. B. Walters, Langmuir, 2014, 30, 6858–6866 CrossRef CAS PubMed.
- V. Gerber, M. King, D. A. Schneider and N. E. Robinson, Equine Vet. J., 2000, 32, 411–417 CrossRef CAS.
- W. Hofmann and B. Asgharian, Toxicol. Sci., 2003, 73, 448–456 CrossRef CAS PubMed.
- D. E. Leith, J. P. Butler, S. L. Sneddon and J. D. Brain, in Comprehensive Physiology, John Wiley & Sons, Inc., 2011 Search PubMed.
- G. W. Burgreen, R. Hester, B. Soni, D. Thompson, D. K. Walters and K. Walters, in Advances in Computational Biology, Springer, 2010, pp. 573–584 Search PubMed.
- A. Courouce-Malblanc, G. Fortier, S. Pronost, B. Siliart and G. Brachet, Vet. J., 2008, 175, 227–233 CrossRef CAS PubMed.
- M. Dawson, D. Wirtz and J. Hanes, J. Biol. Chem., 2003, 278, 50393–50401 CrossRef CAS PubMed.
- R. G. Larson, The structure and rheology of complex fluids, Oxford University Press, New York, 1999 Search PubMed.
- K. Hyun, M. Wilhelm, C. O. Klein, K. S. Cho, J. G. Nam, K. H. Ahn, S. J. Lee, R. H. Ewoldt and G. H. McKinley, Prog. Polym. Sci., 2011, 36, 1697–1753 CrossRef CAS PubMed.
- R. H. Ewoldt, A. E. Hosoi and G. H. McKinley, J. Rheol., 2008, 52, 1427–1458 CrossRef CAS.
- K. S. Cho, K. Hyun, K. H. Ahn and S. J. Lee, J. Rheol., 2005, 49, 747–758 CrossRef CAS.
- R. H. Ewoldt, C. Clasen, A. E. Hosoi and G. H. McKinley, Soft Matter, 2007, 3, 634–643 RSC.
- R. H. Ewoldt, P. Winter, G. H. McKinley, MITlaos version 2.1 Beta for MATLAB (computer software). Cambridge, MA, 2007 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental conditions for mucus collection and separation methods, dried lung mucus morphology (ESI 1), solid content analysis (ESI 2), and experimental details for rheological characterization and additional rheological results from SAOS and LAOS experiments (ESI 3 and ESI 4). See DOI: 10.1039/c4ra05055j |
| This journal is © The Royal Society of Chemistry 2014 |