A highly efficient ligand exchange reaction on gold nanoparticles: preserving their size, shape and colloidal stability†
Abstract
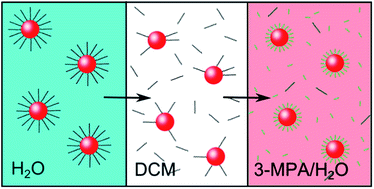
This study presents a new ligand exchange method for cetyl trimethylammonium bromide/chloride (CTAB/C) stabilised gold nanoparticles. It has been shown that the resulting thiol-coated nanoparticles remained colloidally stable in aqueous dispersion and that particle size and morphology was not affected. Furthermore, we were able to achieve nearly complete ligand exchange.


 Please wait while we load your content...
Please wait while we load your content...