A facile and practical one-pot synthesis of [1,2,4]triazolo[4,3-a]pyridines†
Kamlesh S. Vadagaonkara,
Kaliyappan Muruganb,
Atul C. Chaskar*a and
Prakash M. Bhate*a
aDepartment of Dyestuff Technology, Institute of Chemical Technology, Mumbai 400019, India. E-mail: achaskar25@gmail.com; pm.bhate@ictmumbai.edu.in; Fax: +91-22-3361 1020; Tel: +91-22-3361 1111 Tel: +91-22-3361 2707
bNational Taiwan University, Taipei 10617, Taiwan
First published on 30th July 2014
Abstract
A mild, efficient and operationally simple one-pot synthesis of substituted [1,2,4]triazolo[4,3-a]pyridines at room temperature from easily available 2-hydrazinopyridine and substituted aromatic aldehydes has been developed. This functional group tolerant and atom-economic method provides facile access to synthetically and biologically important triazolopyridine derivatives.
Introduction
The triazolopyridine scaffold is present in several heterocyclic compounds1 that possess a broad spectrum of biological and pharmaceutical activities, such as herbicidal, antifungal, anticonvulsant, anxiolytic, antibacterial, antithrombotic, anti-inflammatory and antiproliferative.2–5In view of the importance of triazolopyridines in pharmacology and functional materials, a ready access to libraries of these derivatives is highly desirable. On the background of aforementioned applications of triazolopyridine derivatives, numerous methods have been developed for its synthesis. Along with 2-hydrazinopyridine either aldehyde or acid is often employed as a precursor in presence of reagents such as phosphorous oxychloride,6 lead tetra-acetate,7 bromine,8 PS-PPh3/CCl4 under microwave,9 (diacetoxy)iodobenzene,3a bis(trifluoroacetoxy) iodobenzene10 etc. In addition to this acylation of 2-hydrazinopyridine followed by dehydrative cyclization is also widely used method for synthesis of 1,2,4-triazolo[4,3-a]pyridines.11
Since large scale synthesis is concerned, besides potential utility the aforementioned methods are associated with some shortcomings such as use of harsh reaction condition, toxic reagents, high temperature, longer reaction time, high equivalent of the reagents and formation of large amount of toxic by-products.
Recently Schmidt et al. synthesized triazolopyridines from 2-hydrazinopyridine and imidates under mild condition at 50–70 °C by using 1.5 equiv. of acetic acid, wherein the reaction rate is governed by electronic and steric properties of the hydrazine and imidate moieties.12 Thiel et al.13 synthesized triazolopyridines from 2-chloropyridine and aldehyde hydrazones, in a two step protocol involving Pd catalyzed intermolecular coupling followed by oxidative cyclization with chloramine T. The use of expensive palladium catalyst and basic aqueous work up to remove the toluenesulfonamide formed during the course of the reaction limits the use of this method for large scale synthesis. Reichelt and co-workers14 accomplished the synthesis of triazolopyridines by using acid hydrazide and 2-chloropyridine. They used palladium catalyst along with an array of phosphine ligands for the reaction of acid hydrazide with 2-chloropyridine followed by dehydrative cyclization in acetic acid under microwave irradiation.
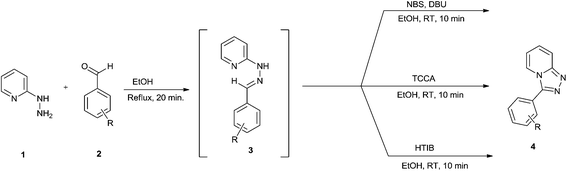
In continuation with our interest in the development of environment friendly protocols for the synthesis of heterocyclic compounds,15–18 we envisaged to access pharmaceutically important triazolopyridine derivatives. In this context, herein we report the synthesis of triazolopyridines by using such reagents as N-bromosuccinimide (NBS)-1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), trichloroisocyanuric acid (TCCA) and hydroxy(tosyloxy)iodobenzene (HTIB) at room temperature (Scheme 1).
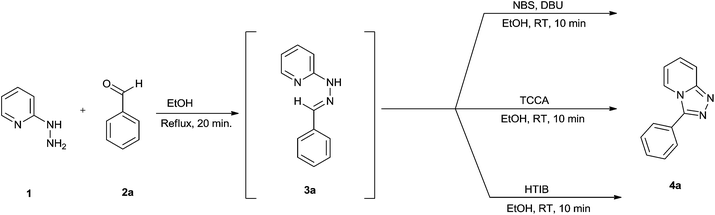
In order to optimize reaction conditions for the one-pot oxidative cyclization we selected the reaction between 2-hydrazinopyridine and benzaldehyde as a model. Initially, these two substrates were heated in EtOH at reflux temperature for 20 minutes. After the completion of the reaction, as indicated by TLC, the reaction mass was cooled to room temperature to afford the heterocyclic hydrazone 3a. Different reagents were then added separately to this reaction mass at room temperature and resulting mixture was stirred for appropriate time. When 1.0 equiv. N-bromosuccinimide (Table 1, entry 1) was used, triazolopyridine 4a was obtained in 57% yield in 10 minutes. We screened different oxidants such as TBHP, Oxone and DMP in combination with N-bromosuccinimide (Table 1, entries 2–4) and were pleasantly surprised when we obtained 4a in 92% yield in presence of 1.0 equiv. of NBS and 1.0 equiv. DBU (Table 1, entry 5). The reaction with DMP as an oxidant afforded a moderate yield of 71% while reactions by using oxone and TBHP resulted in slightly lower yields of 65 and 62%, respectively.
| Entry | Reagentb | Oxidantc | Temp. (°C) | Time (min) | Yield (%)d |
|---|---|---|---|---|---|
| a Reaction conditions: 2-hydrazinopyridine 1 (1.0 equiv.), benzaldehyde 2a (1.0 equiv.).b Reagent (1.0 equiv.).c Oxidant (1.0 equiv.) in EtOH for 10–120 min. at RT-80 °C temp.d Isolated yields. | |||||
| 1 | NBS | — | RT | 10 | 57 |
| 2 | NBS | TBHP | RT | 10 | 62 |
| 3 | NBS | Oxone | RT | 10 | 65 |
| 4 | NBS | DMP | RT | 10 | 71 |
| 5 | NBS-DBU | — | RT | 10 | 92 |
| 6 | I2 | — | RT | 120 | 15 |
| 7 | I2 | TBHP | RT | 120 | 29 |
| 8 | I2 | TBHP | 80 | 10 | 45 |
| 9 | I2 | TBHP | 80 | 120 | 72 |
| 10 | I2 | Oxone | RT | 120 | 35 |
| 11 | I2 | Oxone | 80 | 10 | 52 |
| 12 | I2 | Oxone | 80 | 120 | 76 |
| 13 | I2 | DMP | RT | 10 | 48 |
| 14 | I2-DBU | — | RT | 10 | 67 |
| 15 | KI | Oxone | RT | 120 | 18 |
| 16 | TBAI | Oxone | RT | 120 | 28 |
| 17 | TCCA | — | RT | 10 | 85 |
| 18 | HTIB | — | RT | 10 | 90 |
When 1.0 equiv. of iodine was used as a reagent at room temperature the reaction did not proceed to completion even after 120 minutes and gave only 15% yield (Table 1, entry 6). Use of TBHP (1.0 equiv.) as an oxidant at room temperature resulted in 29% yield with prolonged reaction time (Table 1, entry 7). When the reaction temperature was increased to 80 °C, 72% yield was obtained (Table 1, entry 9) in 120 minutes. The reaction was incomplete when oxone was the oxidant at room temperature, whereas yields of 52% and 76% were obtained when the reaction was carried out at 80 °C for 10 and 120 minutes, respectively (Table 1, entries 11 and 12). A moderate yield of 48% was obtained when DMP was used as an oxidant (Table 1, entry 13). Use of DBU afforded a yield of 67% (Table 1, entry 14). The combination of KI and TBAI with oxone at room temperature resulted in very low yields (Table 1, entries 15 and 16).
A high yield of 85% was obtained in 10 minutes (Table 1, entry 17) when we carried out this reaction in presence of trichloroisocyanuric acid (TCCA) at room temperature. We also tried this reaction by using hydroxy(tosyloxy)iodobenzene (HTIB) and obtained a yield of 90% at room temperature in 10 minutes (Table 1, entry 18). We conclude from Table 1 that the use of 1.0 equiv. of NBS-DBU/TCCA/HTIB separately at room temperature affords 1,2,4-triazolo[4,3-a]pyridine derivatives via a one-pot oxidative cyclization of heterocyclic hydrazone, formed by the condensation of 2-hydrazinopyridine and benzaldehyde in EtOH.
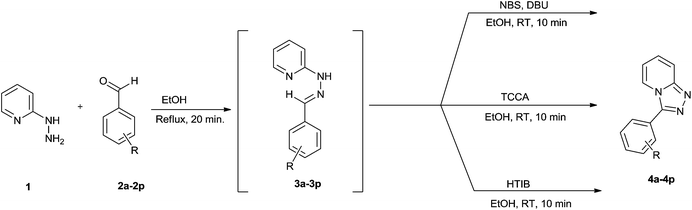
With optimized conditions in hand, we next investigated the scope and limitation of this one-pot oxidative cyclization by using different aromatic aldehydes (Table 2). The yields obtained by using all the three reagents were found to be more or less the same. The reaction is robust and is not affected by nature of substituents.
| Entry | Substrate | Product | Yield (%)c | ||
|---|---|---|---|---|---|
| NBS-DBUb | TCCAb | HTIBb | |||
| a Reaction conditions: 2-hydrazinopyridine 1 (1.0 equiv.), substituted aromatic aldehyde 2 (1.0 equiv.).b Reagent (1.0 equiv.) in EtOH at RT.c Isolated yields. | |||||
| 1 |  |
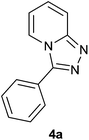 |
92 | 85 | 90 |
| 2 | 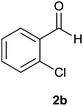 |
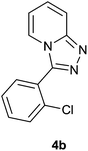 |
87 | 81 | 85 |
| 3 | 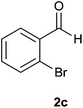 |
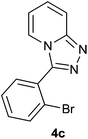 |
90 | 84 | 88 |
| 4 | 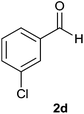 |
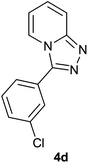 |
88 | 83 | 86 |
| 5 | 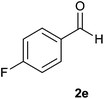 |
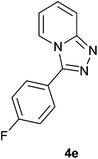 |
89 | 84 | 87 |
| 6 | 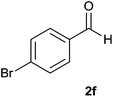 |
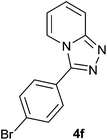 |
94 | 88 | 92 |
| 7 | 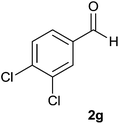 |
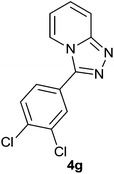 |
90 | 85 | 87 |
| 8 | 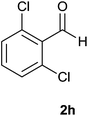 |
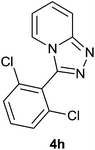 |
85 | 80 | 82 |
| 9 | 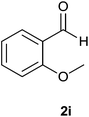 |
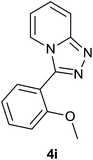 |
86 | 80 | 83 |
| 10 | 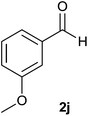 |
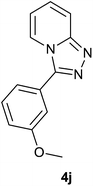 |
88 | 82 | 86 |
| 11 | 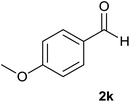 |
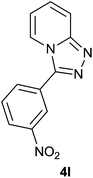
 |
83 | 78 | 80 |
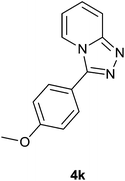
| 12 | 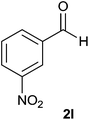 |
 |
94 | 89 | 93 |
| 13 | 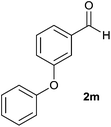 |
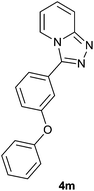 |
88 | 82 | 85 |
| 14 | 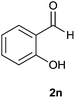 |
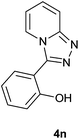 |
89 | 84 | 87 |
| 15 | 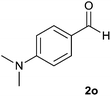 |
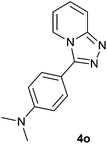 |
87 | 82 | 86 |
| 16 | 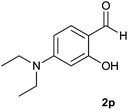 |
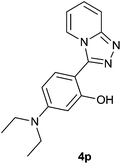 |
85 | 81 | 85 |
The aldehydes bearing halogen substituents at the ortho position of the formyl group (2b, 2c and 2h) due to steric hindrance resulted in slightly lower yields of products as compared to halogen groups present at the meta and para positions (2d–2g). When the aldehydes bearing electron donating methoxy substituent at the ortho, meta and para positions with respect to the formyl group were subjected for oxidative cyclization, the reactivity order of m > o > p was observed. The proof of concept is the excellent yield of 88% for aldehyde 2m with m-phenoxy substituent. Further the aldehydes 2n–2p bearing electron donating groups such as –OH, –NMe2, –NEt2 at the ortho and para position of formyl group resulted in good to excellent yields (81–89%) of the products 4n–4p by using all the three reagents. Albeit, the aldehyde 2l bearing an electron withdrawing NO2 substituent gave best yield due to the enhanced electrophilicity of carbonyl group.
Experimental section
General information
Chemical reagents were obtained from commercial companies. All reactions were performed in a round bottom flask and monitored by TLC performed on aluminum plates (0.25 mm, E. Merck) precoated with silica gel Merck 60 F-254. Developed TLC plates were visualized under a short-wavelength UV lamp. Yields refer to spectroscopically (1H, 13C NMR) homogeneous material obtained after column chromatography performed on silica gel (100–200 mesh size) supplied by S. D. Fine Chemicals Limited, India. 1H and 13C NMR spectra were recorded on Brüker 400 and Varian 300 MHz spectrometers by using tetramethylsilane (TMS) as an internal standard. Coupling constants (J) were measured in Hertz. All chemical shifts are quoted in ppm, relative to CDCl3, by using the residual solvent peak as a reference standard. The number of protons (n) for a given resonance is indicated by nH. High resolution mass spectra (HRMS) were obtained by using positive electrospray ionization (ESI) by Time of Flight (TOF) method. Melting points were recorded on a standard melting point apparatus from Sunder Industrial Product, Mumbai and uncorrected.General procedure for one-pot oxidative cyclization
A mixture of 2-hydrazinopyridine 1 (1.0 mmol), substituted aromatic aldehyde 2 (1.0 mmol) and EtOH (5 mL) was boiled under reflux for 20 minutes to afford the corresponding heterocyclic hydrazone 3. The reaction mixture was cooled to room temperature and to it was added NBS (1.0 mmol) and DBU (1.0 mmol)/TCCA (1.0 mmol)/HTIB (1.0 mmol). The resulting mixture was stirred at room temperature for 10 minutes. After completion of reaction, as indicated by TLC, the EtOH was evaporated under vacuum. Water (10 mL) was added and the mixture extracted with DCM (2 × 10 mL). The combined organic layer was washed successively with 10% sodium bicarbonate solution (10 mL), 10% sodium bisulphate solution (10 mL) and water (10 mL). The organic layer obtained was dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure to give crude product which was purified with column chromatography on 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 mesh silica gel by using 5% MeOH in DCM as the eluent to afford the pure product 4.
200 mesh silica gel by using 5% MeOH in DCM as the eluent to afford the pure product 4.
Product characterization
Conclusion
We have discovered an efficient and elegant straight forward strategy for the synthesis of triazolopyridine derivatives in good to excellent yields at room temperature from easily available 2-hydrazinopyridine and substituted aromatic aldehydes. A simple open flask reaction operation, use of simple and abundant substrates, wide functional group tolerance, mild conditions and short reaction time are striking features of this reaction. Application of this facile and practical protocol for the selective construction of triazolopyridines scaffold in the complex molecules bearing more than one active sites and their eventual use in optoelectronic devices is underway in our group.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by CSIR-New Delhi (sanction no. 01(2427)/10/EMR-II dated 28/12/2010, pool scheme no. 8644-A). K.S.V. thanks UGC-SAP, New Delhi for award of Senior Research Fellowship.References
- G. Jones, Adv. Heterocycl. Chem., 2002, 83, 1–70 CrossRef CAS
.
-
(a) K. F. McClure, Y. A. Abramov, E. R. Laird, J. T. Barberia, W. Cai, T. J. Carty, S. R. Cortina, D. E. Danley, A. J. Dipesa, K. M. Donahue, M. A. Dombroski, N. C. Elliott, C. A. Gabel, S. Han, T. R. Hynes, P. K. Lemotte, M. N. Mansour, E. S. Marr, M. A. Letavic, J. Pandit, D. B. Ripin, F. J. Sweeney, D. Tan and Y. Tao, J. Med. Chem., 2005, 48, 5728–5737 CrossRef CAS PubMed
; (b) D. Kim, L. Wang, J. J. Hale, C. L. Lynch, R. J. Budhu, M. Maccoss, S. G. Mills, L. Malkowitz, S. L. Gould, J. A. DeMartino, M. S. Springer, D. Hazuda, M. Miller, J. Kessler, R. C. Hrin, G. Carver, A. Carella, K. Henry, J. Lineberger, W. A. Schleif and E. A. Emini, Bioorg. Med. Chem. Lett., 2005, 15, 2129–2134 CrossRef CAS PubMed
; (c) L. Savini, L. Chiasserini, C. Pellerano, W. Filippelli and G. Falcone, Farmaco, 2001, 56, 939–945 CrossRef CAS
.
-
(a) D. Brown and Y. Iwai, Aust. J. Chem., 1979, 32, 2727–2733 CrossRef CAS
; (b) A. K. Sadana, Y. Mirza, K. R. Aneja and O. Prakash, Eur. J. Med. Chem., 2003, 38, 533–536 CrossRef CAS
.
-
(a) R. Peignier, A. Chene, R. Cantegril and J. Mortier, Eur. Pat. Appl., 1991, 441718 Search PubMed
; (b) R. Sarges, H. R. Howard, R. G. Browne, L. A. Lebel, P. A. Seymour and B. K. Koe, J. Med. Chem., 1990, 33, 2240–2254 CrossRef CAS
.
-
(a) Y. Yoshimura, K. Tomimatsu, T. Nishimura, A. Miyake and N. Hashimoto, J. Antibiot., 1992, 45, 721–734 CrossRef CAS
; (b) E. C. Lawson, W. J. Hoekstra, M. F. Addo, P. Andrade-Gordon, B. P. Damiano, J. A. Kauffman, J. A. Mitchell and B. E. Maryanoff, Bioorg. Med. Chem. Lett., 2001, 11, 2619–2622 CrossRef CAS
; (c) A. S. Kalgutkar, H. L. Hatch, F. Kosea, H. T. Nguyen, E. F. Choo, K. F. McClure, T. J. Taylor, K. R. Henne, A. V. Kuperman, M. A. Dombroski and M. A. Letavic, Biopharm. Drug Dispos., 2006, 27, 371–386 CrossRef CAS PubMed
.
- J. D. Bower and F. P. Doyle, J. Chem. Soc., 1957, 727–732 RSC
.
- A. Pollak and M. Tišler, Tetrahedron, 1966, 22, 2073–2079 CrossRef CAS
.
- M. S. Gibson, Tetrahedron, 1963, 19, 1587–1589 CrossRef CAS
.
- Y. Wang, K. Sarris, D. R. Sauer and S. W. Djuric, Tetrahedron Lett., 2007, 48, 2237–2240 CrossRef CAS PubMed
.
-
(a) V. S. Padalkar, V. S. Patil, K. R. Phatangare, P. G. Umape and N. Sekar, Synth. Commun., 2011, 41, 925–938 CrossRef CAS
; (b) P. Kumar, Chem. Heterocycl. Compd., 2012, 47, 1237–1243 CrossRef CAS
.
-
(a) D. R. Sliskovic, Bicyclic 5-6 Systems with One Ring Junction, Nitrogen Atom: Two Extra Heteroatoms 2: 0, in Comprehensive Heterocyclic Chemistry II: A Review of the Literature 1982–1995: The Structure, Reactions, Synthesis, and Uses of Heterocyclic Compounds, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Pergamon: Oxford, New York, 1996, vol. 8, ch. 13, pp. 367–388 Search PubMed
.
- M. A. Schmidt and X. Qian, Tetrahedron Lett., 2013, 54, 5721–5726 CrossRef CAS PubMed
.
- O. R. Thiel, M. M. Achmatowicz, A. Reichelt and R. D. Larsen, Angew. Chem., Int. Ed., 2010, 49, 8395–8398 CrossRef CAS PubMed
.
- A. Reichelt, J. R. Falsey, R. M. Rzasa, O. R. Thiel, M. M. Achmatowicz, R. D. Larsen and D. Zhang, Org. Lett., 2010, 12, 792–795 CrossRef CAS PubMed
.
- B. Pawar, V. Padalkar, K. Phatangare, S. Nirmalkar and A. Chaskar, Catal. Sci. Technol., 2011, 1, 1641–1644 CAS
.
- K. Phatangare, V. Padalkar, D. Mhatre, K. Patil and A. Chaskar, Synth. Commun., 2009, 39, 4117–4121 CrossRef CAS
.
- S. Takale, S. Parab, K. Phatangare, R. Pisal and A. Chaskar, Catal. Sci. Technol., 2011, 1, 1128–1132 CAS
.
- S. Takale, J. Patil, V. Padalkar, R. Pisal and A. Chaskar, J. Braz. Chem. Soc., 2012, 23, 966–969 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra04961f |
| This journal is © The Royal Society of Chemistry 2014 |