Synthesis and characterization of F-doped nanocrystalline Li4Ti5O12/C compounds for lithium-ion batteries
Xiaona Hana,
Zhen Zhaoa,
Yunlong Xu*a,
Dong Liub,
Huang Zhanga and
Chongjun Zhaoa
aKey Laboratory for Ultrafine Materials of Ministry of Education, Shanghai Key Laboratory of Advanced Polymeric Materials, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, P.R. China. E-mail: happyhxn@163.com; xuyunlong@ecust.edu.cn; Fax: +86-021-64250838; Tel: +86-021-64252019
bDepartment of Materials Science and Engineering, University of Texas at Arlington, Arlington, TX 76019, USA
First published on 19th August 2014
Abstract
The F-doped and carbon coated Li4Ti5O12−xFx/C (LTOF/C) composite anode materials are synthesized via a conventional solid-state reaction with lithium citric acid as the conductive carbon source, and LiF as the fluorine source. The structure and electrochemical properties of the LTOF/C materials are investigated and the effects of carbon coating and F ion doping on the performance of as-prepared materials are discussed. Under a potential range from 0.01 to 2.5 V, LTOF/C exhibits better reversibility and higher cyclic stability compared with LTO and LTO/C, especially at high current rates. The initial specific discharge capacity is 325.6 mA h g−1 at a current density of 170 mA g−1, and remains about 165 mA h g−1 over 1000 cycles when the current density increases to 850 mA g−1. The main factors influencing the excellent performances should be attributed to the cooperation of the F-doping and carbon-coating, which can increase the amount of mixed Ti3+/Ti4+, leading to improved ionic conductivity and electron conductivity in LTO at the same time.
Introduction
Lithium-ion batteries continue to play a critical role in the development of modern portable digital and wireless technologies.1 Their commercial success has triggered efforts to further expand their use to large-scale energy-storage applications, such as electric drive vehicles and grid storage.2,3As one of the most promising anode candidates for rechargeable Li-ion batteries, the spinel Li4Ti5O12 (LTO) material has attracted considerable attention. It presents many advantages compared to commercial carbon anodes: a high lithiation plateau around 1.5 V (vs. Li/Li+) that avoids the safety problem caused by the formation of metallic Li on the anode,4–6 relatively high specific capacity (175 mA h g−1), excellent cyclic reversibility and stability due to its zero-strain at charge/discharge.7 However, the main obstacle that impedes widespread applications of Li4Ti5O12 is its poor electronic conductivity (ca.10−13 S cm−1) and ionic conductivity (ca. 10−8 S cm−1). Consequently, the material shows inferior electrochemical performance at high current rates.8
Various approaches have been explored in attempt to improve the conductivity of Li4Ti5O12, such as reducing the particle size, surface-coating a conductive substance as carbon, doping, and hybridization.9–14 Among these approaches, carbon-coating has been demonstrated very useful in elevating poor electronic conductivity of Li4Ti5O12.15 Ion-doping would reduce accessible Li-ion diffusion path of the Li4Ti5O12.16 Nevertheless, in order to optimize the rate capability, it is necessary for a comprehensive consideration of both electronic conductivity and ionic diffusivity.17 Gao et al. synthesized a spherical La-doped Li4Ti5O12/C composite material by the outer gel technique.18 But the La-doped Li4Ti5O12/C exhibits an unsatisfied rate performance.
In our previous work, it has been demonstrated that the rate capacity and cyclic stability of LTO can be significantly enhanced by either carbon coating at the particle surface or doping F into O2− site.19,20 In this work, two approaches are combined and studied in a systematic manner, aiming at improving electronic conductivity and ionic conductivity of LTO material simultaneously by employing synergistic effect of conductive layer coating and non-metal element doping.
Experimental section
Sample preparation
The bare spherical Li4Ti5O12 (LTO) was synthesized by high-energy ball milling Li2CO3 (AR, Shanghai Chemical Agents Co. Ltd) and anatase TiO2 (AR, Nanjing High Technology Nano Material Co. Ltd) with Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti molar ratio of 0.84
Ti molar ratio of 0.84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 6 h. Ethyl alcohol was used as dispersant. The mixture was then spray-dried for sphere-making and annealed at 800 °C for 12 h under ambient atmosphere to achieve well-crystallized Li4Ti5O12 powder. Li4Ti5O12/C and Li4Ti5O12−xFx/C (x = 0.3) samples were synthesized in the similar fashion. Note that designated amount of F was chosen for optimal electrochemical performance of the battery according to our previous work.19 Proper amount of lithium citric acid (AR, Shanghai China Lithium Industrial Co., Ltd.) and LiF (AR, Aladdin Industrial Inc.) were used in couple with Li2CO3 and TiO2 as raw materials for high energy ball milling. Lithium citric acid was used as carbon source and lithium source. And LiF was used as fluorine source and lithium source at the same time. All samples were then annealed at the same temperature in argon. The obtained samples are denoted as LTO/C and LTOF/C, respectively.
1 for 6 h. Ethyl alcohol was used as dispersant. The mixture was then spray-dried for sphere-making and annealed at 800 °C for 12 h under ambient atmosphere to achieve well-crystallized Li4Ti5O12 powder. Li4Ti5O12/C and Li4Ti5O12−xFx/C (x = 0.3) samples were synthesized in the similar fashion. Note that designated amount of F was chosen for optimal electrochemical performance of the battery according to our previous work.19 Proper amount of lithium citric acid (AR, Shanghai China Lithium Industrial Co., Ltd.) and LiF (AR, Aladdin Industrial Inc.) were used in couple with Li2CO3 and TiO2 as raw materials for high energy ball milling. Lithium citric acid was used as carbon source and lithium source. And LiF was used as fluorine source and lithium source at the same time. All samples were then annealed at the same temperature in argon. The obtained samples are denoted as LTO/C and LTOF/C, respectively.
Sample characterization
In the LTOF/C sample, the final stoichiometry of the F−-substituted spinel compounds were confirmed by composition analysis. The content of F− ion was analyzed using anion selective electrode, and the content of titanium was determined by titration with EDTA while the determination of Li+ were performed on a VISTA AX simultaneous ICP-OES spectrometer with axial view configuration (Varian, Australia). And comparison with the pure sample LTO.The crystal structures of the as-prepared samples were characterized by X-ray diffraction (XRD, D/Max 2500 V, Japan) using Cu Kα radiation (λ = 0.15418 nm). The thermogravimetric analysis (TGA, STA 449 F3, Germany) was conducted on the sample to analyze its weight change during the heat treatment. The sample was heated from room temperature to 1000 °C with a heating rate of 5 °C min−1 under air flow of 60 mL min−1. The surface morphology and microstructure of the sample were characterized by scanning electron microscope (SEM, Hitachi S-4800, Japan) and transmission electron microscopy (TEM, JEOL-2100, Japan). The chemical state at the surface of the sample was analyzed by X-ray photoelectron spectroscopy (XPS, Perkin-Elmer PHI-5000C multifunctional XPS, USA).
Electrochemical measurements
The working electrode compound was fabricated by dispersing 85 wt% active materials, 5 wt% acetylene black (AB, Shanghai Haohua Chemical Co. Ltd), and 10 wt% polyvinylidene fluoride (PVDF, Shanghai Ofluorine Chemical Technology Co. Ltd) in N-methyl-2-pyrrolidone (NMP, Shanghai Lingfeng Chemical Reagent) solvent to create a slurry. The slurry was then coated onto the aluminum foil and dried at 120 °C over 10 h. The working electrode was made by cutting the aluminum foil into circular pieces with a diameter of 8 mm after the drying. Coin-type cell CR2032 was used to test the battery performance. The cell was assembled in an Argon-filled glove box (Super1220/750, Mikrouna China Co. Ltd) using lithium metal as anode electrode, a polypropylene micro-porous film (Cellgard2400) as the separator, 1 M LiPF6 in ethylene carbonate (EC) and diethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Guangzhou Tianci Material Technology Co. Ltd) as the electrolyte and then left aging at least 12 h before any test was performed.
1, v/v) (Guangzhou Tianci Material Technology Co. Ltd) as the electrolyte and then left aging at least 12 h before any test was performed.
The charge/discharge cycling test was performed on a battery test instrument (CT2001A, LAND Battery Program-control Test System, China) over a voltage range of 0.01–2.5 V at room temperature. The electrochemical impedance spectroscopy (EIS) was performed on the cell using a CHI660D electrochemical station. The signals were potentiostatically measured at the cell's open circuit voltage (OCV) with an AC oscillation of 5 mV amplitude over the frequencies from 105 Hz to 10−2 Hz.
Results and discussion
On the basis of chemical analysis, the final chemical formulate of the F− substituted spinel compounds were determined as Li3.57Ti5O12.01, Li3.62Ti5O11.88F0.11/C, respectively. The results are shown in Table 1. As shown in Table 1, parts of lithium and fluorine were lost due to their volatilization during the synthesis at high temperature.| Sample no. | Nominal formula | Composition (wt%) | The final chemical composition | |||
|---|---|---|---|---|---|---|
| Li | F | Ti | O | |||
| LTO | Li4Ti5O12 | 5.41 | 52.03 | 41.66 | Li3.57Ti5O12.01 | |
| LTOF/C | Li4Ti5O11.7F0.3/C | 5.51 | 0.45 | 52.12 | 41.29 | Li3.62Ti5O11.88F0.11/C |
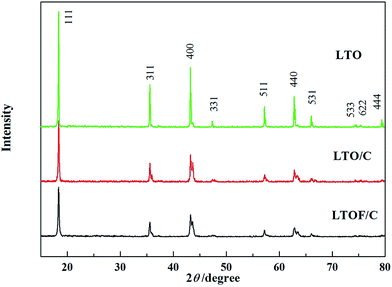
Fig. 1 shows XRD patterns of pristine LTO, LTO/C and LTOF/C samples. All three samples show major peaks of cubic LTO (JCPDS no. 49-0207). Carbon is not detected maybe as the carbon in the samples is amorphous and in low content, which is further verified by TG analysis. No other impurity or second phase is found. This indicates that F-doping and carbon-coating do not cause any detectable crystal structure change. We calculated lattice parameters of three samples and the results are present in Table 2. It indicates that the lattice parameters increase with carbon coating and/or F-doping. The slight lattice parameter increase of LTO/C sample is believed due to the presence of more Ti3+ ions in the lattice while carbon reduces Ti4+ ions to Ti3+ ions as a reducing agent. Since the ionic radius of Ti4+ (0.75 Å) is smaller than that of Ti3+ (0.81 Å),21 the lattice parameter of LTO/C sample increases. On the other side, the further lattice parameter growth of LTOF/C sample indicates that fluorine doping can expand lattice parameter more. Although this observation on our sample may contradict with Vegard's rule that predicts a lattice parameter reduction when replacing larger O2− ions with smaller F− ions, we ascribe such inconsistency to lattice charge rebalance caused by doping. When F− ions substitute O2− ions in LTO crystal lattice, charge imbalance is created as F− ions are less negatively charged than O2− ions. To accommodate such, more Ti3+ (either reduced by carbon and/or F doping) must be produced in the lattice to re-reaching charge rebalance. As a result, lattice parameter of the sample increases.22
| Sample | α (nm) |
|---|---|
| LTO | 0.8362 |
| LTO/C | 0.8369 |
| LTOF/C | 0.8373 |
In order to prove the presence of carbon and determine the content of it, TG analysis was further conducted on LTO/C and LTOF/C samples and the results are shown in Fig. 2. And Fig. 2 reveals the presence of carbon and further analysis indicates that the both samples contain about 7.5 wt% carbon in them. This result also shows that the carbon is in low content, and explains why the XRD can not detect the presence of carbon. What's more, the same content of carbon is beneficial to the further comparison of the LTO/C and LTOF/C samples.
 | ||
| Fig. 2 TGA curves of the LTO/C (a) and LTOF/C (b) samples measured from 20 to 1000 °C under an air flow of 60 mL min−1 with a heating rate of 5 °C min−1. | ||
Fig. 3 shows SEM images of LTO, LTOF/C and LTO/C samples. It can be seen from Fig. 3(a), (c) and (e) that LTO, LTOF/C and LTO/C samples are all composed of well-dispersed spherical primary particles and the size of these particles are about 12 μm, 24 μm and 20 μm, respectively. In Fig. 3(b), (d) and (f), it is clearly seen that these primary particles are agglomerates of extensive small secondary particles in both samples. Comparing Fig. 3(b) with Fig. 3(d) and (f), we can clearly find that nanoparticles around 50 nm are encompassed in LTOF/C and LTO/C samples, which are much smaller than the secondary particles in LTO sample. It is concluded that the results exhibit that the carbon from the pyrolysis of lithium citric acid may can help inhibit the growth of particles during the annealing sintering process. This is favored because smaller particles give rise to higher conductivity and thus better electrochemical performance of the material as the diffusion length of charge carriers is shortened.17,23
 | ||
| Fig. 3 SEM images of LTO, LTOF/C and LTO/C samples for the micron-size secondary particles (a), (c) and (e). And their nanosized primary particles (b), (d) and (f), respectively. | ||
In order to investigate elementary distribution of different elements in LTOF/C sample, EDS mapping was performed on one primary particle and the results are shown in Fig. 4. The mapping results demonstrate appreciable amount of carbon and fluorine are uniformly dispersed within the particle and carbon mapping result is in accordance with TGA analysis. Alternatively, the mapping results are rendered as evidence of successful carbon coating and F doping through high temperature solid state reactions.
To further study the distribution of surface coated carbon within the secondary particle in LTOF/C and LTO/C samples, TEM measurement was performed with the results shown in Fig. 5. As shown in Fig. 5(a) and (c), carbon forms a coating layer that continuously surrounds at the surface of secondary nano particles, which enables a conductive network through out the whole material. The HRTEM image in Fig. 5(b) and (d) reveals that the coated carbon layer has a thickness of about ca. 2 nm. Combined with SEM results, such thin layer carbon network on LTO particle surface is expected to (i) significantly inhibit the growth of primary particles and (ii) greatly facilitate the transport of charge carriers.
Fig. 6 shows XPS survey and high resolution spectra of LTO and LTOF/C samples. The survey spectra in Fig. 6(a) identifies strong signals of C, O and Ti in LTO sample and F in addition to three same elements in LTOF/C sample. The peak at 284.08 eV is ascribed to the amorphous carbon coated on the surface of the particles. LTOF/C sample exhibits one F 1s peak centered at 685.53 eV in Fig. 6(b) and it may be attributed to the Li–F bond. The binding energy for Ti 2p3/2 presents a shift of 1.7 eV to the position of Ti3+ (457.8 eV)24 after doping as revealed in Fig. 6(c), suggesting the existence of a mixed Ti4+/Ti3+valence of Ti ions.25 These results corroborate with our XRD and EDS analysis, confirming the substitution of O2− ions by F− ions and reduction of Ti4+ ions in the crystal lattice.
In order to investigate the influence of different voltage range on the samples, The charge/discharge cycling test was respectively performed at a voltage range of 0.01–2.5 V and 1.0–2.5 V. Fig. 7(a) and (b) show the first charge and discharge curves of the pristine LTO, LTO/C and LTOF/C with voltage range of 0.01–2.5 V (a) and 1.0–2.5 V (b) at the current density of 170 mA g−1. In Fig. 7(a), the first discharge/charge specific capacity of LTO, LTO/C and LTOF/C samples are 146.7 mA h g−1/145.4 mA h g−1, 164.5 mA h g−1/161.1 mA h g−1, 169.6 mA h g−1/164.9 mA h g−1, respectively. At a voltage range of 0.01–2.5 V in Fig. 7(b), the first discharge/charge specific capacity of LTO, LTO/C and LTOF/C samples are 205.5 mA h g−1/181.2 mA h g−1, 281.6 mA h g−1/224.8 mA h g−1, 325.6 mA h g−1/260.5 mA h g−1, respectively. The first discharge/charge specific capacity is much higher when the cut-off voltage is set between 0.01 V and 2.5 V.
Fig. 7(c) shows the cycling performance of the three samples with the current density of 170 mA g−1 and voltage range of 1.0–2.5 V over 50 cycles. Both LTO/C and LTOF/C samples show much higher specific capacity compared to pristine LTO sample. But LTOF/C sample exhibits the most excellent electrochemical performance. Fig. 7(d) shows the cycling performance of LTOF/C with different voltage range over 10 cycles at the current density of 170 mA g−1. After 10 cycles, the capacity of LTOF/C at the voltage range of 0.01–2.5 V remains 230 mA h g−1, which is much higher than that at the voltage range of 1.0–2.5 V. It is corroborated by ab initio calculations showing that it is possible to obtain an 8a Li occupation in an all 16c framework up to a theoretical composition of Li8.5Ti5O12 when the applied potential is as low as 0.01 V, which provides a theoretical capacity that is about 1.5 times higher than that of the compound lithiated to Li7Ti5O12 by means of first-principles calculations.26 From the first discharge/charge specific capacity and the cycling performance at different cut-off voltage can find that the preferred voltage range is 0.01–2.5 V.
Fig. 8 shows initial charge/discharge behavior of the prepared pristine LTO, LTO/C and LTOF/C samples under different current densities. The cut-off voltage is set between 0.01 V and 2.5 V. Both LTO/C and LTOF/C samples show much higher specific capacity under various current densities compared to pristine LTO sample. But LTOF/C sample exhibits the most excellent electrochemical performance. Under low current density (170 mA g−1), the first discharge/charge specific capacity of LTO, LTO/C and LTOF/C samples are 205.5 mA h g−1/181.2 mA h g−1, 281.6 mA h g−1/224.8 mA h g−1, 325.6 mA h g−1/260.5 mA h g−1, respectively. All the three samples exceed the theoretical capacity of Li4Ti5O12 (175 mA h g−1), which is due to the two-phase redox reaction between Li4Ti5O12 and Li8.5Ti5O12.27,28 Under higher current densities, all three samples show deteriorated electrochemical performance due to severely restrained ionic transport. However, LTO/C and LTOF/C samples are capable of suppressing capacity loss more compared to pristine LTO sample and such ability is especially manifested by LTOF/C sample. For LTOF/C sample, we ascribe the capacity improvement/retention to the synergistic effect of surface carbon coating and F-doping. On one hand, the thin layer carbon coating facilitates fast transport of charge carriers thus boosts electronic conductivity of the material while F-doping increases the lattice parameter of the material thus broadens the diffusion pathway of Li+ ion during its insertion/extraction.29 On the other hand, either F-doping and/or carbon coating create excessive Ti3+ ions in the lattice, which is in favor of further improving the conductivity the material.
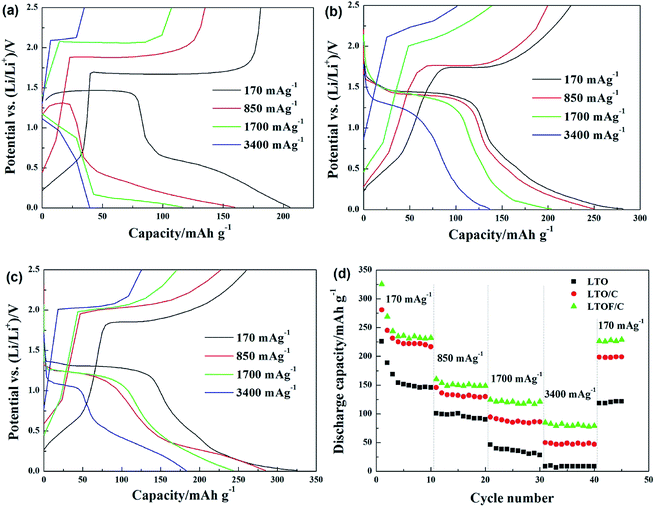 | ||
| Fig. 8 The first charge and discharge curves of the pristine LTO (a), LTO/C (b) and LTOF/C (c) at different current rates. The rate performances of the three samples (d). | ||
Furthermore, rate performance of three samples were compared in Fig. 8(d) and no distinguishable capacity decline are observed for LTO/C and LTOF/C samples. Note that the irreversible capacity fading on all samples after first discharge are due to the formation of SEI film at the surface of electrode when the cut-off voltage is blew 0.7 V.30 The SEI film may be contributed from the decomposed of electrolyte in this low voltage range and the side reactions such as solid electrolyte interface (SEI) layer formation on the surface of lithium titanate particles. And the organic lithium alkylcarbonates were the primary components of SEI film formed during the reduction process.
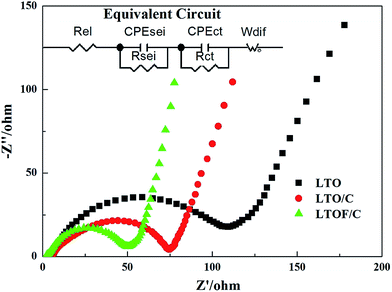
Fig. 9 shows the AC impedance spectra of the three samples, which were measured at the stable voltage of 1.55 V, respectively. AC impedance spectra are fitted using an equivalent circuit in the Fig. 9. In this equivalent circuit, Rel, Rsei and Rct are the resistance of electrolyte, the resistance of solid electrolyte interphase film and charge transfer resistance, respectively. CPE is placed to represent the double layer capacitance and passivation film capacitance. W represents the Warburg impedance. And the fitted data was calculated with ZView2.0 software and the results were shown in the Table 3. From the table, Rel and Rsei of the three samples are almost the same. Comparing the three samples, it can be found that LTOF/C samples have a lower Rct, and a smaller value of W, indicating that the F-doping and carbon coating could enhance the Li-ion diffusion and decrease the charge transfer resistance, which is beneficial for the electrochemical performance of the electrodes. This result is in agreement with the discharge curves in Fig. 8. As a result, the F-doping can facilitate the charge-transfer reaction of the Li4Ti5O12 electrodes, and the F-doped Li4Ti5O12/C has a higher capacity and better cycling stability at high charge/discharge rates.
| Sample | Rel (Ω) | Rsei (Ω) | Rct (Ω) | W (Ω−1 s−1) |
|---|---|---|---|---|
| a Rel: electrolyte resistance; Rsei: SEI film resistance; Rct: charge transfer resistance; W: Warburg diffusion impedance. | ||||
| LTO | 6.02 | 10.12 | 68.79 | 83.18 |
| LTO/C | 5.37 | 10.97 | 44.81 | 48.78 |
| LTOF/C | 5.12 | 9.39 | 39.25 | 39.32 |
Fig. 10 shows the cycling performance and coulombic efficiency of LTOF/C sample at the current density of 850 mA g−1 over 1000 cycles. The columbic efficiency over the 1000 cycles is close to 100%. Such excellent performance is mainly attributed to small particle size, surface coating and F-doping, which improves the conductivity, shortens the ionic diffusion distance and provides a thermodynamically more stable system.
 | ||
| Fig. 10 Graph of specific charge/discharge capacities and coulombic efficiency vs. cycle number for (LTOF/C)/Li cells at a current density of 850 mA g−1. | ||
Conclusions
In this paper, we have successfully synthesized the spherical F-doped Li4Ti5O12/C powders with nanocrystalline by conventional solid-state reaction. The products possess good morphology and appropriate particle size. After F-doping and carbon-coating in the Li4Ti5O12, the grain size is smaller. When the cut-off voltage is from 0.01 to 2.5 V, the F-doped Li4Ti5O12/C has a high capacity and good cycling stability at high charge/discharge rates. Owing to the cooperation effect of substituting F− into O2− sites and compounding with carbon, it can increase the amount of Ti3+/Ti4+ mixing as charge compensation and not only decrease the charge transfer resistance of Li4Ti5O12, but also improve the lithium ion diffusion and electronic conductivity at the same time. As a result, LTOF/C sample exhibits the first discharge capacity as high as 325.6 mA h g−1 at the current density of 170 mA g−1. Even at a current density of 850 mA g−1, the specific discharge capacity still remains 165 mA h g−1 over 1000 cycles. All the results suggest that it should be a better method of doping F− and compounding with carbon at the same time to improve the cycle stability and rate performance of the Li4Ti5O12 material.Acknowledgements
This work is supported by Shanghai Leading Academic Discipline Project (B502) and Shanghai Key Laboratory Project (08DZ2230500).Notes and references
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- F. T. Wagner, B. Lakshmanan and M. F. Mathias, J. Phys. Chem. Lett., 2010, 1, 2204–2219 CrossRef CAS.
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- C. J. Kim, N. S. Norberg, C. T. Alexander, R. Kostecki and J. Cabana, Adv. Funct. Mater., 2013, 23, 1214–1222 CrossRef CAS PubMed.
- W. Wang, H. L. Wang, S. B. Wang, Y. J. Hu, Q. X. Tian and S. Q. Jiao, J. Power Sources, 2013, 228, 244–249 CrossRef CAS PubMed.
- B. Zhang, Z. D. Huang, S. W. Oh and J. K. Kim, J. Power Sources, 2011, 196, 10692–10697 CrossRef CAS PubMed.
- T. Ohzuku, A. Ueda and N. Yamamoto, J. Electrochem. Soc., 1995, 142, 1431–1435 CrossRef CAS PubMed.
- D. Su, F. Wang, C. Ma and N. Jiang, Nano Energy, 2013, 2, 343–350 CrossRef CAS PubMed.
- W. J. H. Borghols, M. Wagemaker and U. Lafont, J. Am. Chem. Soc., 2009, 131, 17786–17792 CrossRef CAS PubMed.
- G. N. Zhu, C. X. Wang and Y. Y. Xia, J. Electrochem. Soc., 2011, 158, 102–109 CrossRef PubMed.
- A. S. Prakash, P. Manikandan, K. Ramesha, M. Sathiya, J. M. Tarascon and A. K. Shukla, Chem. Mater., 2010, 22, 2857–2863 CrossRef CAS.
- Q. Y. Zhang, C. L. Zhang, B. Li, S. F. Kang, X. Li and Y. J. Wang, Electrochim. Acta, 2013, 98, 146–152 CrossRef CAS PubMed.
- Y. J. Bai, C. Gong, N. Lun and Y. X. Qi, J. Mater. Chem. A, 2013, 1, 89–96 CAS.
- B. Zhang, Y. S. Liu, Z. D. Huang, S. Oh, Y. Yu, Y. W. Mai and J. K. Kim, J. Mater. Chem., 2012, 22, 12133–12140 RSC.
- L. Cheng, X. L. Li, H. J. Liu, H. M. Xiong, P. W. Zhang and Y. Y. Xia, J. Electrochem. Soc., 2007, 154, 692–697 CrossRef PubMed.
- Y. Qi, Y. Huang, D. Jia, S. J. Bao and Z. P. Guo, Electrochim. Acta, 2009, 54, 4772–4776 CrossRef CAS PubMed.
- G. N. Zhu, Y. G. Wang and Y. Y. Xia, Energy Environ. Sci., 2012, 5, 6652–6667 CAS.
- J. Gao, C. Y. Jiang and C. R. Wan, J. Electrochem. Soc., 2010, 157, 39–42 CrossRef PubMed.
- Z. Zhao, Y. L. Xu, M. D. Ji and H. Zhang, Electrochim. Acta, 2013, 109, 645–650 CrossRef CAS PubMed.
- S. W. Zheng, Y. L. Xu, C. J. Zhao, H. K. Liu, X. Z. Qian and J. H. Wang, Mater. Lett., 2012, 68, 2–35 Search PubMed.
- J. Wolfenstine, U. Lee and J. L. Allen, J. Power Sources, 2006, 154, 287–289 CrossRef CAS PubMed.
- S. Scharner, W. Weppner and P. Schmid-Beurmann, J. Electrochem. Soc., 1999, 146, 857–861 CrossRef CAS PubMed.
- A. S. Prakash, P. Manikandan, K. Ramesha, M. Sathiya, J. M. Tarascon and A. K. Shukla, Chem. Mater., 2010, 22, 2857 CrossRef CAS.
- F. Werfel and O. Brummer, Phys. Scr., 1983, 28, 92–96 CrossRef CAS.
- Y. K. Sun, S. T. Myung, B. C. Park, J. Prakash, I. Belharouak and K. Amine, Nat. Mater., 2009, 8, 320–324 CrossRef CAS PubMed.
- Z. Zhong, C. Ouyang, S. Shi and M. Lei, ChemPhysChem, 2008, 9, 2104–2108 CrossRef CAS PubMed.
- H. Ge, N. Li, D. Y. Li, C. S. Dai and D. L. Wang, J. Phys. Chem. C, 2009, 113, 6324–6326 CAS.
- M. Venkateswarlu, C. H. Chena, J. S. Dob, C. W. Lin, T. C. Chou and B. J. Hwang, J. Power Sources, 2005, 146, 204–208 CrossRef CAS PubMed.
- Y. D. Huang, R. R. Jiang, S. J. Bao, Z. F. Dong, Y. L. Cao, D. Z. Jia and Z. P. Guo, J. Solid State Electrochem., 2009, 13, 799–805 CrossRef CAS.
- T. F. Yi, Y. Xie, Y. R. Zhu, R. S. Zhu and H. Y. Shen, J. Power Sources, 2013, 222, 448–454 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |