Pyrolysis behavior of biomass with different Ca-based additives
Li Zhang*ab,
Bo Zhangab,
Zhongqing Yangab and
Yunfei Yanab
aKey Laboratory of Low-grade Energy Utilization Technologies and Systems (Chongqing University), Ministry of Education of China, Chongqing, 400044, P.R.China. E-mail: lizhang_cqu@163.com; Fax: +86-23-65102107; Tel: +86 023-65103114
bCollege of Power Engineering, Chongqing University, Chongqing, 400044, P.R.China
First published on 21st July 2014
Abstract
CaO is an important catalyst in biomass gasification and pyrolysis. However, CaO can stem from many precursors. And, the effect between different precursors has still not been studied. Calcined natural limestone, natural dolomite, calcium oxide, calcium carbonate, calcium acetate, and calcium propionate were used to investigate their pyrolysis reactivity of biomass (peanut shells and pine sawdust) at high temperature (>800 °C). Experiments were conducted using a thermogravimetric apparatus and a fixed-bed pyrolysis system. Pine sawdust displayed higher pyrolysis reactivity than peanut shells. During pyrolysis, Ca-based additives fixed carbon dioxide mainly in the temperature range of 300–700 °C. By addition of Ca-based additives, the concentrations of hydrogen and carbon monoxide were increased and the concentrations of methane and carbon dioxide (over 18 vol%) were decreased. De-carbonation started around 700 °C. The emission of CO2 by de-carbonation promoted the reduction of char by the Boudouard reaction and methane by the dry reforming reaction. The total pyrolysis conversion decreased as PI-Dol > PI-CaA > PI-Lime > PI-Ca > PI-CaP > PI Raw > PI-CaC. The excellent pyrolysis reactivity of biomass in the presence of calcined calcium acetate can be ascribed to its preeminent physical structure. Addition of Ca-based additives increased the activation energy of the main devolatilization region. The activation energy of biomass with calcined natural dolomite, about 110 kJ mol−1, was much lower than that of other Ca-based additives studied at high temperatures. Pyrolysis of the pure biomass sample can be simply hypothesized as a first order reaction, but it varied significantly with the addition of additives.
1. Introduction
Fossil fuels are currently the most important energy sources,1 but the reserves of fossil fuels are finite. With energy consumption increasing, there is an urgent need for long-term alternative energy sources. Biomass which plays an irreplaceable role in the energy world is an eco-friendly alternative source. It is a renewable and abundantly available source, and its utilization is now receiving great attention for such advantages.Hydrogen (H2) is an environment-friendly fuel, and it is being used in various transports, industrial, commercial and residential applications, where fossil fuels are currently used. It is an important gas and attracted intensive research globally in recent years. Gasification of biomass is a promising way to produce rich hydrogen gas, for its product mainly consists of hydrogen (H2) and carbon monoxide (CO).2
The previous research on the steam gasification of biomass, without CO2 capture, achieved H2 concentrations in the product gas of only 40–50 vol%. When coupled with CO2 capture, the output of H2 from biomass gasification was reported to increase to ∼80 vol%.3 CaO plays an active role in situ CO2 capture during gasification.4 CaO reacts with CO2 to form CaCO3 (eqn (1)), which drives the water-gas shift reaction (eqn (2)) and water-gas reaction (eqn (3)) to produce more hydrogen. On the other hand, the heat released from carbonation reaction also favors hydrogen production.5 Moreover, CaO is reported to be a promising catalyst to elimination of tar,6–8 and reduce of tar also creates additional hydrogen.
| CO2 + CaO ↔ CaCO3 | (1) |
| CO + H2O ↔ CO2 + H2 | (2) |
| C + H2O → CO + H2O | (3) |
Wei et.al.9 used calcined limestone as a CO2 absorbent during gasification of pine sawdust, which increased the extent of the water-gas shift reaction and enhanced the yield of H2. In their latter work, they found that gasification of legume straw with calcined dolomite owns highest H2 yield (46 vol%) compared with that with calcined limestone and olivine in a free-fall reactor.10 Acharya et.al.11 got a hydrogen concentration up to 54.43 vol% and a drop of 93.33 vol% in carbon dioxide when calcined CaCO3 was used in biomass gasification as an additive. Except for gasification, the effect of CaO was also investigated in reforming of methane for hydrogen production. Martavaltzi et.al.12 used the CaO–Ca12Al14O33 (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 wt) which was synthesized using calcium acetate as a CaO precursor acted as an effective CO2 sorbent for steam reforming of methane and hydrogen with concentrations higher than 92 vol% was achieved.
15 wt) which was synthesized using calcium acetate as a CaO precursor acted as an effective CO2 sorbent for steam reforming of methane and hydrogen with concentrations higher than 92 vol% was achieved.
Biomass pyrolysis is an essential pre-step of both combustion and gasification processes.13 It was reported that addition of analytical reagent CaO could enhance the gaseous production and slightly increased the production of H2 from pyrolysis of biomass.14 Nevertheless, the effect of CaO in the pyrolysis of biomass has received limited in-depth study. As we know, calcium precursor plays an important role in the production of calcium-based sorbents with better performance for CO2 capture.15 However, in pyrolysis process, the role of CaO is not simply as sorbent. And, the performance of CaO from different precursors for CO2 capture may be totally different for the situation of biomass pyrolysis. To date, there is still no work in literature that compares the different calcium precursors and it is not clear whether there are any other potentially better precursors. Moreover, few studies have been reported the effect of Ca-based additive on the pyrolysis kinetics of biomass. Therefore, the objective of this work is to explore the performance of various Ca-based additives produced from different precursors on the biomass pyrolysis and their kinetic characteristics, aiming to identify the best sorbent precursors. Two natural stones and four calcium precursors were used in this paper including, natural limestone, natural dolomite, calcium oxide, calcium carbonate, calcium acetate, and calcium propionate.
2. Material and methods
2.1 Feedstock and additives
Two kinds of biomass, pine sawdust and peanut shell, were used in the present work. The pine tree is common worldwide, and is often used in manufacturing of solid wood furniture for its rapid growth rate and good wood quality. Pine sawdust used in this study is from a furniture factory. Peanut shell is somehow a waste, for we only have the peanut for food. Pine sawdust and peanut shell were identified as PI and PE. Prior to TGA (Thermogravimetric apparatus) experiments, all biomass samples were manually pre-ground to particles that were less than about 120 mesh in diameter and then dried in an oven at 104 °C for 24 hours to provide a basis for experiments and analysis. The elemental analysis (C, H, N, O and S) of the wood was carried out using an Elemental Analyzer Vario ELIII, while the oxygen content was determined by the difference. The properties of biomass samples are listed in Table 1. The volatile content of pine sawdust and peanut shell was 77.38 and 69.44%, supporting the use of them as energy source. In Table 1, “others” includes ash, some benzene–alcohol and hot-water extractives.| Proximate analysis | Elemental analysis | Composition of lignocelluloses material | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Vad | FCad | Aad | Cad | Had | Oad | Sad | Nad | Cellulose | Hemicellulose | Lignin | Others | |
| PI | 8.78 | 77.38 | 11.76 | 2.08 | 46.67 | 6.98 | 38.53 | 0.36 | 0.24 | 48.75 | 20.24 | 22.72 | 8.29 |
| PE | 8.61 | 69.44 | 14.43 | 7.52 | 45.52 | 6.46 | 46.68 | 0.25 | 0.89 | 30.12 | 35.18 | 14.26 | 20.44 |
The natural dolomite and limestone were obtained from Hebei Province of China. The compositions of dolomite and limestone are listed in Table 2. Other precursors were analytical reagent with more than 99.9% purity (Sinopharm Chemical Reagent Beijing Co., Ltd.). Ca-based additives were prepared by calcination of the precursors in the muffle furnace at 900 °C for 30 min. All the additives were ground and sieved 60 to 100 mesh. Six additives were blended with biomass samples by dry-mix before tests, respectively, and checked with scanning electron microscopy (SEM) to ensure the thorough mixing. The amount of Ca-based additive added was 2.5 mg (50 wt% of biomass sample). PI-Dol represents the pine sawdust sample added with calcined dolomite, similarly, PI-Lime for calcined limestone, PI-Ca for CaO, PI-CaC for calcined CaCO3, PI-CaA for calcined calcium acetate, PI-CaP for calcined calcium propionate and so on throughout this paper.
| CaO | MgO | Al2O3 | Fe2O3 | SiO2 | Na2O | Others | Loss on ignition | |
|---|---|---|---|---|---|---|---|---|
| Dolomite | 30.23 | 20.68 | 0.42 | 0.093 | 3.10 | 0.011 | 0.306 | 45.16 |
| Limestone | 78.10 | 0.98 | 0.11 | 0.032 | 1.56 | — | 0.438 | 19.78 |
2.2 Additive characterization
The morphologies of the prepared additives were observed using a scanning electron microscope (SEM, TESCAN VEGA 3, 10 kV). Nitrogen adsorption–desorption measurements were carried out on a Micromeritics ASAP-2010 sorptometer at liquid nitrogen temperature. Each additive was degassed at 473 K and <1.5 mPa for 3 h before measurement. The specific surface areas were calculated by the BET (Brunner–Emmet–Teller) method and pore volumes and pore sizes were evaluated from the desorption branch of the isotherm based on the BJH (Barrett–Joyner–Halenda) model.2.3 Pyrolysis experiments
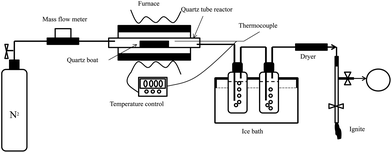
All pyrolysis experiments were conducted using a thermogravimetric analyzer (STA409 PC, Germany). The sample was placed in a ceramic crucible which is 70 ml in volume and 6 mm in diameter. About 5 mg of biomass was used for each experiment run. The heating temperature in the TG was started from ambient temperature to 900 °C at a heating rate of 20 K min−1. Nitrogen at a fixed flow rate of 50 ml min−1 was used as a carrying gas in the TG throughout the study to ensure an inert environment. And 20 ml min−1 of nitrogen was selected as protection gas. The changes of sample weight and temperature were continually detected and recorded during the experiment. During each experiment, the TGA recorded several thousand data points. For analysis, a smooth function was fitted to the data and then numbers of data points were generated from the smoothed data set over equal intervals from T = 40 °C to T = 900 °C. The disturbing effects of the transport processes were diminished by low sample masses and moderate heating rate. All the experiment runs with any given condition were usually carried out more than twice.A schematic diagram of the fixed-bed pyrolysis reaction system is shown in Fig. 1.
It was used to study the property of gas production from the biomass pyrolysis. In order to lessen the heat and mass transfer resistance, only 3 g of biomass and 1.5 g of additive were used for each test. The reaction tube, which is made of quartz, is 1200 mm length and 30 mm outer diameter. The quartz boat is 350 mm length. And stainless steel sheathed type K (3 mm diameter, 600 mm length) thermocouple is used, covering the temperature range of 0–1200 °C. The tests were performed under atmospheric pressure and 10 ml min−1 of N2. The temperature was risen from ambient temperature to 850 °C at a heating rate of 19 °C min−1. The gaseous products were collected by the 10 l sample bag during the temperature range of 200–850 °C, and then off-line analyzed by gas chromatography equipped with the TCD detector. After each test, the residue in the quartz boat was carefully collected and precisely weighted.
2.4 Pyrolysis kinetics
The non-isothermal kinetics for solid decomposition reaction can be written as follow:
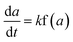 | (4) |
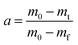 | (5) |
In eqn (5), m0, mt and mf represent the initial, instantaneous and final weights of the sample, respectively. The rate constant k can be expressed by the Arrhenius equation:
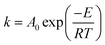 | (6) |
| f(a) = (1 − a)n | (7) |
Plugging eqn (6) and eqn (7) into eqn (4), we have:
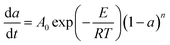 | (8) |
Then, the differential methods were introduced to determine the kinetic parameters, activation energy, pre-exponential factor, and the reaction order of biomass pyrolysis.
For β = dT/dt, eqn (8) can be re-written as:
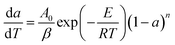 | (9) |
Then, taking a natural logarithm (ln) on both sides of eqn (9) gives:
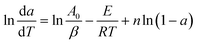 | (10) |
In the above equation, A0, E and n are assumed to be constant in a given temperature range. And da/dT can be directly obtained from the DTG (Differential Thermogravimetry) curve. For two random points (a1, T1) and (a2, T2), which are very close to each other on the DTG curve, two functions are obtained:
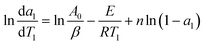 | (11) |
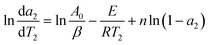 | (12) |
Subtracting eqn (11) with eqn (12) we obtain:
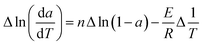 | (13) |
A more brief equation is shown by rearrange eqn (13):
 | (14) |
The plot of Δln(da/dT)/Δln(1 − a) versus Δ(1/T)/Δln(1 − a) for a series of points becomes a linear line. Accordingly, the slope of the fitting straight line equal to E/R and the activation energy E can be determined. Meanwhile, the reaction order n equals to the intercept of the regression line. Pre-exponential factor A0 can be calculated from eqn (11) or eqn (12) using the parameters E and n obtained above.
3. Results and discussion
3.1 Pyrolysis characteristics of raw biomass samples
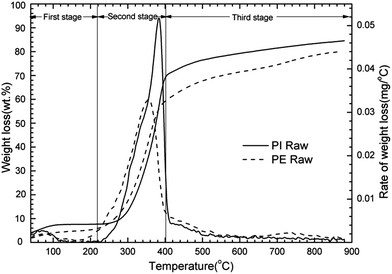
The pyrolysis properties of raw PE and PI samples are shown in Fig. 2.From Fig. 2, according to the decomposition rate, pyrolysis process was divided into three stages approximately. The characteristic parameters of pyrolysis are listed in Table 3. It demonstrated that the main weight loss appeared at the second stage for both samples. The volatile released during the second stage was 76.4% and 68% of the final volatile matter released for PI and PE sample, respectively, and the decomposition intensity of PI sample was higher. The onset temperature of main devolatilization (the temperature that the DTG line starts rising) was about 180 °C for PE sample, but it was slightly higher for PI sample, approximately 230 °C. Moreover, the maximum decomposition rate for PI sample was found to shift to about 20 °C higher temperature compared with that of PE sample. The final temperature of main devolatilization was around 415 °C for both PI and PE sample. The final weight loss of PI sample was 80.6%, while 78.2% for PE sample. The different decomposition characteristics during between two biomass samples were caused by their different chemical and physical compositions.16 Pyrolysis of hemicellulose, cellulose, and lignin occurred in the following temperature ranges: 150–315, 315–400 and 250–900 °C, respectively.17 Hemicellulose decomposes earlier, and then higher hemicellulose content in PE may leads to its lower onset temperature of devolatilization. Moreover, higher amount of cellulose and lignin in PI sample implies that higher temperature is needed to decompose. Higher volatile matters content favors the decomposition of PI sample. Higher ash and fixed carbon content in PE sample leads to its higher residual mass.
| Sample | Characteristics temperatures (°C) | Mass loss (%) | |||||
|---|---|---|---|---|---|---|---|
| To | Tm | Tf | 40–220 °C | 220–400 °C | 400–880 °C | 40–880 °C | |
| PI raw | 230 | 381 | 415 | 3.8 | 61.6 | 14.8 | 80.6 |
| PE raw | 180 | 361 | 412 | 4 | 53.2 | 21 | 78.2 |
3.2 Additive characterization
Fig. 3 shows the morphology of the Ca-based additives after calcination observed from the scanning electron micrograph. | ||
| Fig. 3 SEM images of (a) natural dolomite, (b) natural limestone, (c) calcium carbonate and (d) calcium acetate which were calcined in muffle furnace at 900 °C for 30 min in air. | ||
From Fig. 3, it showed that all samples after calcination presented a porosity structure. However, the calcined calcium carbonate is different, from its SEM image, that it may display a macropore or almost non-porous structure. The microstructure of calcined dolomite is compact, and the pores in it are like channels. The distribution of calcined limestone grains is more regular, and the shape of limestone particle is like cylinder with short length and diameter of around 0.5 μm. Meanwhile, the microstructure of calcined calcium acetate is similar with that of calcined limestone, but shows less regular distribution. Textural properties are summarized in Table 4. Surface area and pore volume of calcined CaCO3 and CaO were much lower than others. Average pore size of calcined CaCO3 is largest (44.43 nm), which indicates many of macropores in it. The calcined dolomite exhibited highest surface area of ca. 14.933 m2 g−1 and a pore volume of 0.1193 cm3 g−1. Calcined limestone, Ca(Ac)2 and Ca(Pr)2 showed comparable surface area, but the average pore size was much smaller for calcined Ca(Ac)2.
| Sample | SBET (m2 g−1) | Pore volume (cm3 g−1) | Average pore size (nm) |
|---|---|---|---|
| Calcined dolomite | 14.93 | 0.119 | 31.96 |
| Calcined limestone | 4.89 | 0.016 | 14.84 |
| Calcined CaCO3 | 1.24 | 0.003 | 44.43 |
| Calcined Ca(Ac)2 | 4.60 | 0.008 | 8.46 |
| Calcined CaO | 1.06 | 0.002 | 13.08 |
| Calcined Ca(Pr)2 | 4.37 | 0.006 | 11.91 |
3.3 Effect of calcined natural stones on pyrolysis characteristics of biomass
The pyrolysis properties of PE and PI samples with calcined dolomite and limestone are presented in Fig. 4. Detail pyrolysis information is listed in Table 5. The whole process for PI and PE sample with additive clearly presented four-stage thermal degradation, which was different from that of pure biomass samples.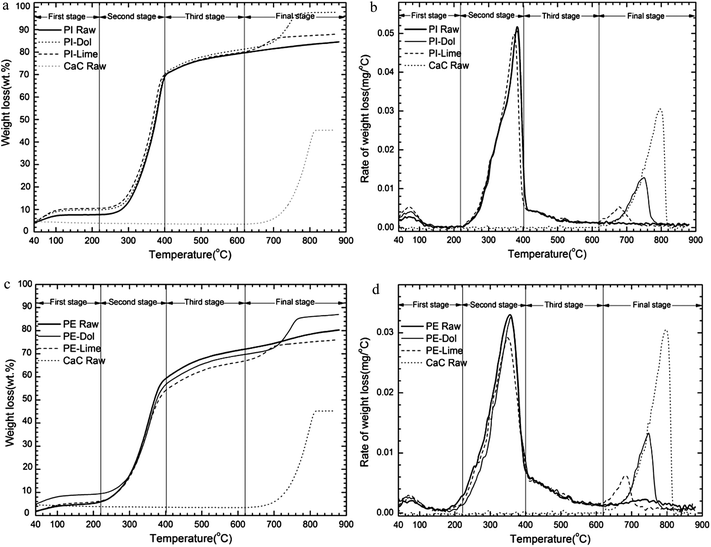 | ||
| Fig. 4 Pyrolysis properties of PI and PE sample with/without calcined rocks. (a) TG results of PI sample, (b) DTG results of PI sample, (c) TG results of PE sample, (d) DTG results of PE sample. | ||
| Sample | Characteristics temperatures (°C) | Mass loss (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| To | Tm | Tf | T′o | T′m | T′f | 40–220 °C | 220–400 °C | 400–620 °C | 620–880 °C | 40–880 °C | |
| PI raw | 230 | 381 | 415 | — | — | — | 3.8 | 61.6 | 10.4 | 4.8 | 80.6 |
| PI-Dol | 231 | 380 | 416 | 656 | 753 | 780 | 5.2 | 60.4 | 11.2 | 17.4 | 94.2 |
| PI-Lime | 229 | 376 | 416 | 620 | 680 | 720 | 6.2 | 59.6 | 10 | 7.8 | 83.6 |
| PE raw | 180 | 361 | 412 | — | — | — | 4 | 53.2 | 12.8 | 8.2 | 78.2 |
| PE-Dol | 184 | 362 | 410 | 667 | 749 | 783 | 4.6 | 47.2 | 13 | 17.2 | 82 |
| PE-Lime | 182 | 357 | 411 | 612 | 683 | 718 | 4.6 | 48 | 12.8 | 9 | 74.4 |
| Pure CaCO3 | — | — | — | 660 | 800 | 818 | — | — | — | — | — |
| CaO + H2O ↔ Ca(OH)2 | (15) |
| Ca(OH)2 + CO2 → CaCO3 + H2O | (16) |
From Fig. 4b and d, it can be seen that sample with additive showed another fast decompose region compared with that of raw sample, which located in the temperature range of 620–880 °C. The fast loss of weight could partially due to the calcination of calcium carbonate which was formed during the pyrolysis.3 On the other hand, in order to better understand the effect of CaO on pyrolysis properties of biomass sample, we conducted the calcination of CaCO3 alone under the same condition as the biomass pyrolysis. It clearly displays in Table 5 that the onset temperature of calcium carbonate decomposition, around 680 °C, is consistent with that of both PI and PE sample with calcined dolomite. However, it is higher than that of samples with calcined limestone (around 620 °C). Ohtsuka and Tomita reported that calcium is apt to disperse on char, and on the carbon surface, CaCO3 started to decompose at a lower temperature.18 Hence, higher calcium content in the limestone probably resulted in a lower decomposition temperature of CaCO3 when co-pyrolysis with char. The temperature for maximum reaction rate was the same for both PE and PI sample with calcined limestone, around 680 °C, but was much lower than that with calcined dolomite (approximately 750 °C for both kinds of biomass). The end temperature was identical for PI and PE sample with calcined limestone, about 60 °C lower than that with calcined dolomite (approximately 780 °C for both PI and PE sample). The pyrolysis characteristic temperatures of different kind of biomass with the same additive in the fourth stage were identical, which was different with that of the main devolatilization region. That is because the residue of biomass (almost coke) is much simpler at this higher temperature range than that of initial stages. Compared with raw PE sample, the weight loss was 9% enhanced by adding calcined dolomite, and 0.8% increased by calcined limestone. Compared with raw PI sample, it was increased by about 12.6% due to addition of calcined dolomite, and accordingly 3% improved by adding calcined limestone. Calcined dolomite showed higher pyrolysis reactivity on biomass.
3.4 Influence of CaO from different precursors on pyrolysis of biomass
The pyrolysis profiles of PI sample with or without additives from different precursors are presented in Fig. 5. From Fig. 5b, the temperature for peak decomposition rate in the first stage was consistent with that of sample with calcined natural stones. The main decomposition region was recorded in the second stage.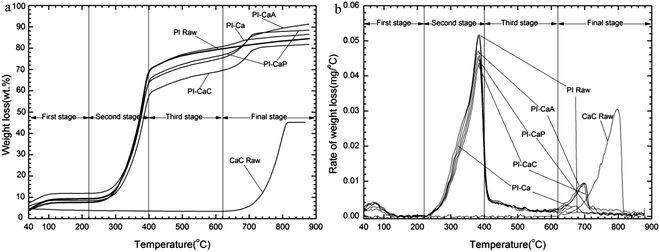 | ||
| Fig. 5 Pyrolysis profiles of PI sample with additives from different precursors. (a) TG results, (b) DTG results. | ||
We can find in Table 6 that the characteristic temperature of the main devolatilization region was almost unchanged by adding Ca-based additives. The onset temperatures of decomposition in the final stage were identical for all samples studied (about 620 °C). The temperature for maximum reaction rate was the same for all samples, except PI-Ca. The final temperature of PI-Ca was lower than that of other samples. Compared with that of sample with calcined natural stones, the weight losses of sample with additive from different precursor were slightly lower during the second stage. That could be resulted from the higher content of CaO in the chemical agents than natural stones, which leads to higher amount of CO2 captured. The mass loss of CaA in the fourth stage was the highest (15.8%) among all the four chemical grade additives studied, and almost three times as much as the none addition one. And the weight losses for samples with additive were all higher than that of raw sample.
| Sample | Characteristics temperatures (°C) | Mass loss (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| To | Tm | Tf | T′o | T′m | T′f | 40–220 °C | 220–400 °C | 400–620 °C | 620–880 °C | 40–880 °C | |
| PI Raw | 230 | 381 | 415 | — | — | — | 3.8 | 61.6 | 10.4 | 4.8 | 80.6 |
| PI-Ca | 231 | 381 | 415 | 621 | 678 | 694 | 5 | 59.4 | 12.2 | 5.8 | 82.4 |
| PI-CaC | 232 | 382 | 415 | 619 | 700 | 718 | 2.6 | 49.4 | 11.8 | 12.2 | 76 |
| PI-CaP | 232 | 382 | 415 | 619 | 702 | 717 | 4.6 | 52.6 | 12.2 | 11.6 | 81 |
| PI-CaA | 232 | 381 | 415 | 620 | 700 | 717 | 5 | 54.2 | 11.6 | 15.8 | 86.6 |
| Pure CaCO3 | — | — | — | 660 | 800 | 818 | — | — | — | — | — |
Total mass loss (40–880 °C) of sample with CaA was the largest (86.6%). For the case of analytical reagent CaO precursors, the different behaviors are largely caused by their different physical characteristics, such as specific surface area, pore size and pore volume etc. For CaA additive, it has the higher surface area and larger pore volume, 4.606 m2 g−1 and 0.0085 m3 g−1, compared with the other three chemical precursors, which should be the main reason leads to its higher CO2 capture ability.19 At the same time, higher surface area provides a large number of chemisorption sites for interaction with CO2 and the gasification medium.
3.5 Kinetics study
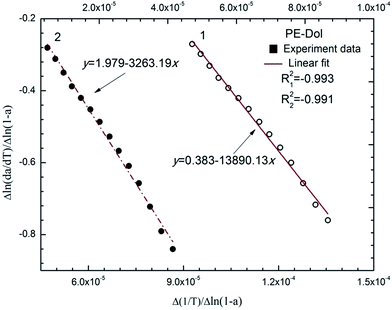
Fig. 6 shows TG, DTG and DSC (Differential Scanning Calorimeter) curves of PE-Dol sample. From the DSC curve, it shows that the whole pyrolysis process is endothermic. The largest heat requirement was in the main pyrolysis region, from 220 °C to 620 °C, after that the heat requirement was declining gradually. The second endothermic peak of DSC curve, which was due to CaCO3 decomposition, tar elimination, and reduction of char, was around 750 °C. The last valley of DSC curve caused no corresponding changes on TG curve. It indicates the physical structure changes of the sample.The kinetic calculations were carried out using eqn (14), based on the assumption that the A0 was a constant in a given temperature range, and we had only done linear fitting to the main decomposition regions of the biomass. The typical plot of Δln(da/dT)/Δln(1 − a) versus Δ(1/T)/Δln(1 − a) of PE-Dol sample is shown in Fig. 7. The line 1 was resulted from linear fitting of the data from the fourth pyrolysis stage, and it was related to bottom horizontal axis. Line 2 was attached to the top horizontal axis and it was the linear fitting line of the second decomposition stage. From the slope of each line, the value of E can be obtained. The order of reaction n is equal to the intercept of the line. When E and n are known, the A0i of the number i point can be calculated by using eqn (9), and then we finally get the average A0 for N points 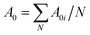 . In this way, the kinetic parameters of all samples were determined and results are presented in Table 7.
. In this way, the kinetic parameters of all samples were determined and results are presented in Table 7.
| Sample | Temp (°C) | (−dM/dt)max (mg min−1) | n | E (kJ mol−1) | A0 (min−1) | −R2 |
|---|---|---|---|---|---|---|
| PI | 300–393 | 1.034 | 0.87 | 43.57 | 6.1 × 103 | 0.988 |
| PI-Lime | 310–385 | 0.992 | 2.17 | 40.20 | 9.8 × 103 | 0.993 |
| 664–701 | 0.122 | 3.13 | 380.2 | 2.9 × 1023 | 0.995 | |
| PI-CaC | 312–402 | 0.872 | 2.00 | 62.88 | 3.79 × 105 | 0.971 |
| 660–750 | 0.185 | 2.18 | 383.10 | 3.7 × 1022 | 0.972 | |
| PI-Ca | 315–415 | 1.025 | 1.80 | 69.90 | 2.19 × 106 | 0.991 |
| 663–680 | 0.058 | 2.25 | 300.56 | 2.25 × 1021 | 0.990 | |
| PI-Dol | 295–400 | 1.011 | 1.98 | 60.76 | 3.2 × 105 | 0.982 |
| 680–790 | 0.256 | 0.40 | 110.93 | 3.6 × 105 | 0.981 | |
| PI-CaP | 303–412 | 0.911 | 2.30 | 69.68 | 2.1 × 106 | 0.993 |
| 640–723 | 0.189 | 2.00 | 327.00 | 9.3 × 1018 | 0.987 | |
| PI-CaA | 297–405 | 0.941 | 3.10 | 84.20 | 5 × 107 | 0.992 |
| 643–712 | 0.190 | 1.70 | 316.80 | 1.6 × 1018 | 0.989 | |
| PE | 280–401 | 0.661 | 1.26 | 21.56 | 1 × 102 | 0.996 |
| PE-Lime | 286–406 | 0.587 | 1.10 | 20.21 | 5.4 × 102 | 0.970 |
| 670–725 | 0.160 | 3.81 | 390 | 2.4 × 1023 | 0.965 | |
| PE-Dol | 316–379 | 0.651 | 1.97 | 27.12 | 2.49 × 102 | 0.991 |
| 720–760 | 0.266 | 0.40 | 114 | 5.43 × 105 | 0.993 |
Many studies on pyrolysis with additives used first order of reaction to conduct the calculation of kinetic parameters. However, it is obvious that the addition of Ca-based additive increased the order of reaction. Hence, only pyrolysis of the pure biomass sample can be treated as the first order reaction. Activation energy of pure PI sample was higher (43.57 kJ mol−1) than PE sample (21.56 kJ mol−1), which meant PE sample was easier to decompose. When added with Ca-based additives, the activation energies for all samples were increased, except that of sample with calcined limestone. Nevertheless, there was a large difference between addition of calcined dolomite and other additives in the fourth stage. Calcined dolomite showed lowest activation energy, only 110.93 kJ mol−1 for PI-Dol and 114 kJ mol−1 for PE-Dol, this was in accordance with their highest decomposition rate. The change of kinetic parameters with addition of Ca-based matter shows that the course and mechanism of biomass pyrolysis may be altered. Moreover, the effects are different for different additives at different temperatures, especially for calcined dolomite.
3.6 Pyrolysis conversion in fixed-bed
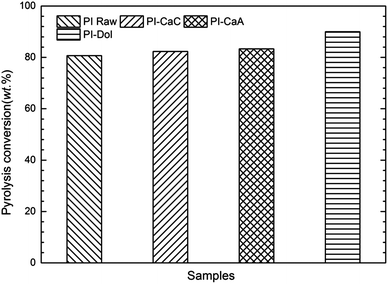
The additives, calcined dolomite, calcium carbonate and calcium acetate, were chosen to further study their effect on pyrolysis of PI sample in the fixed-bed pyrolysis system. The weights of residue in the boat were measured after each test, and the pyrolysis conversions are calculated and shown in Fig. 8.The pyrolysis conversion of PI-Dol was highest. The conversion of PI-Dol was increased by 10% compared with that of PI Raw. And the conversion for the sample with Ca-based additives were higher than that of none addition one, which probably means CaO can increase the carbon conversion during pyrolysis. These results were almost in agreement with that of TGA.
3.7 Distribution of gaseous product
The compositions of gas products with different additives are shown in Fig. 9.With Ca-based additives, the hydrogen concentrations from biomass pyrolysis were all increased by about 10 vol%. The concentrations of carbon monoxide were increased by about 8 vol%. At the same time, it largely lowered the amount of CO2. The highest concentration of hydrogen came from pyrolysis of PI-CaA sample; meanwhile, it owned lowest concentration of CO2. The reason may be that calcined calcium acetate owns better CO2 capture ability than others two additives, which leads to deeper influence on the water shift reaction. The highest concentration of CO came from the pyrolysis of PI-Dol, which was almost 40 vol%. The highest CO amount is believed to be resulted from its stronger catalysis on Boudouard reaction (eqn (17)) and tar reforming reaction (eqn (18) and (19)). These conclusions were in agreement with the TGA information from Section 3.3.2.
| C + CO2 → 2CO | (17) |
| Tar + CO2 → H2 + CO + … | (18) |
| Tar + H2O → H2 + CO + CH4 + … | (19) |
3.8 Gas release profiles
Fig. 10 shows the evolution rate of the gases versus temperature during pyrolysis of PI sample with or without additive.For sample with not additive, it released almost CO2 before 400 °C. Release of CO2 began at a lower temperature, and increased until 600 °C, and then it decreased with temperature. Carbon monoxide largely released after 400 °C. The concentration of CO first increased until 550 °C then decreased gradually, and after 700 °C it almost stable to a constant (around 15 vol%). Concentration of H2 started to release at 450 °C, and gradually increased with temperature.
With additive, the lower concentration of CO2 in temperature range of 300–400 °C can partly prove that the reaction of eqn (15) and (16) were exist. In the temperature range of 500–700 °C, CO concentrations were all lower than none addition one, and CO2 was also much decreased, but the concentration of hydrogen was increased. The reason is that, firstly, CO2 was captured by CaO from Ca-based additive because of carbonation reaction, then, the lowered concentration of CO2 promotes the water shift reaction to the direction of hydrogen production which resulted in production of more H2 and lessen amount of CO. After 700 °C, there shows an increase in CO2, and CO concentration. Concentration of hydrogen increased till 750 °C, but decreased after that. This should be a comprehensive result by considering water gas shift reaction, water gas reaction, Boudouard reaction and tar reforming reactions. Decomposition of CaCO3 let out more CO2. In the meantime, Boudouard reaction consumed CO2 and char, and produce CO. Water gas shift reaction and water gas reaction also changed the distribution of H2 and CO, but with less importance for insufficient of steam. Tar reforming includes dry reforming and steam reforming, both reactions let out mostly CO and H2 at high temperature. The release of CH4 was enhanced by the addition of additives in the temperature range of 400–700 °C, this is based on the catalytic activity of cracking volatile compounds (tar) into light hydrocarbons. After 700 °C, large amount of CO2 enhanced the dry reforming of CH4 (eqn 20), which resulted in reduction of methane.
| CH4 + CO2 → 2H2 + CO | (20) |
4. Conclusions
Experiments were conducted using a thermogravimetric analyzer and a fixed-bed system to investigate the effect of different Ca-based additives on biomass pyrolysis. Several conclusions were made:• With the Ca-based additives addition, all samples displayed another fast weight loss stage, which is mainly due to de-carbonation, and char reduction at elevated temperature. Ca-based additives enhanced the pyrolysis conversion at higher temperatures. Total pyrolysis conversions of both PE and PI samples with calcined dolomite were highest. Followed by sample with calcined calcium acetate. Calcined calcium acetate, because of its good physical structures, favored pyrolysis of biomass.
• The addition of Ca-based additives increased the activation energy and changed the order of reaction. In the second large decomposition region, the activation energy of sample with dolomite was much lower compared with that of CaO derived from analytical reagent, which probably means the different mechanisms.
• CO2 concentration was largely lowered because of carbonation and Boudouard reaction. Over 20 vol. % concentration of CO2 was decreased by addition of calcined calcium acetate. At the same time, hydrogen concentration was raised to 42 vol. %, because of enhanced water gas shift and tar reforming reaction. CO concentration was also increased because of enhanced Boudouard and tar reforming reaction. Dry reforming of CH4 leaded its decrease in concentration at high temperature.
5. Nomenclature
| a | Conversion of sample (−) |
| E | Activation energy (kJ mol−1) |
| t | Heating time (min) |
| R | Universal gas constant (= 8.314 J mol−1 K−1) |
| A0 | Pre-exponential factor (min−1) |
| m | Weight of sample (mg) |
| T | Temperature (K) |
| n | Order of reaction (−) |
| β | Heating rate (K min−1) |
| k | Rate constant (min−1) |
| R2 | Correlation coefficient (−) |
| Vad | Volatile matter content (air-dried basis) |
| Mad | Moisture content (air-dried basis) |
| Aad | Ash content (air-dried basis) |
| FCad | Fixed-carbon content (air-dried basis) |
| Had | Hydrogen content (air-dried basis) |
| Cad | Carbon content (air-dried basis) |
| Sad | Sulfur content (air-dried basis) |
| Oad | Oxygen content (air-dried basis) |
| Tm | Temperature for the maximum rate of devolatilization (°C) |
| Nad | Nitrogen content (air-dried basis) |
| Tf | Final temperature of the main devolatilization (°C) |
| To | Onset temperature of the main devolatilization (°C) |
| T′o | Onset temperature of the second large decomposition (°C) |
| T′m | Temperature for the maximum rate of the second large decomposition (°C) |
| T′f | Final temperature of the second large decomposition (°C) |
Acknowledgements
The authors would like to thank the financial supports of Natural Science Foundation of China with Project no. 51206200, and the Fundamental Research Funds for the Central Universities with Project no. CDJZR12140031, and visiting Scholar Foundation of Key Lab. of Low-grade Energy Utilization Technologies and System, MOE of China in Chongqing University (LLEUTS-201301).References
- International Energy Agency, World Energy, Outlook 2012 Search PubMed.
- Y. Kalinci, A. Hepbasli and I. Dincer, Biomass-based hydrogen production: a review and analysis, Int. J. Hydrogen Energy, 2009, 34, 8799–8817 CrossRef CAS PubMed.
- N. H. Florin and A. T. Harris, Enhanced hydrogen production from biomass with in situ carbon dioxide capture using calcium oxide sorbents, Chem. Eng. Sci., 2008, 63, 287–316 CrossRef CAS PubMed.
- M. R. Mahishi and D. Y. Goswami, An experimental study of hydrogen production by gasification of biomass in the presence of a CO2 sorbent, Int. J. Hydrogen Energy, 2007, 32, 2803–2808 CrossRef CAS PubMed.
- X. Q. Zhao, M. Wang, H. Z. Liu, C. Zhao, C. Y. Ma and Z. L. Song, Effect of temperature and additives on the yields of products and microwave pyrolysis behaviors of wheat straw, J. Anal. Appl. Pyrolysis, 2013, 100, 49–55 CrossRef CAS PubMed.
- J. Han and H. Kim, The reduction and control technology of tar during biomass gasification/pyrolysis: An overview, Renewable Sustainable Energy Rev., 2008, 12, 397–416 CrossRef CAS PubMed.
- T. Hanaoka, T. Yoshida, S. Fujimoto, K. Kamei, M. Harada and Y. Suzuki, et al. Hydrogen production from woody biomass by steam gasification using a CO2 sorbent, Biomass Bioenergy, 2005, 28, 63–68 CrossRef CAS PubMed.
- D. Sutton, B. Kelleher and J. R. H. Ross, Review of literature on catalysts for biomass gasification, Fuel Process. Technol., 2001, 73, 155–173 CrossRef CAS.
- L. G. Wei, S. P. Xu, J. G. Liu, C. H. Liu and S. Q. Liu, Hydrogen Production in Steam Gasification of Biomass with CaO as a CO2 Absorbent, Energy Fuels, 2008, 22, 1997–2004 CrossRef CAS.
- L. G. Wei, S. P. Xu, L. Zhang, C. H. Liu, H. Zhu and S. Q. Liu, Steam gasification of biomass for hydrogen-rich gas in a free-fall reactor, Int. J. Hydrogen Energy, 2007, 32, 24–31 CrossRef CAS PubMed.
- B. Acharya, A. Dutta and P. Basu, An investigation into steam gasification of biomass for hydrogen enriched gas production in presence of CaO, Int. J. Hydrogen Energy, 2010, 35, 1582–1589 CrossRef CAS PubMed.
- C. S. Martavaltzi, E. P. Pampaka, E. S. Korkakaki and A. A. Lemonidou, Hydrogen Production via Steam Reforming of Methane with Simultaneous CO2 Capture over CaO-Ca12Al14O33, Energy Fuels, 2010, 24, 2589–2595 CrossRef CAS.
- C. Somerville, Energy from Biomass; Workshop Presentation for the Inter Academy Council Study Report; Lighting the Way: Towards Sustainable Energy Future, IAC, Amsterdam, the Netherlands, 2005 Search PubMed.
- W. H. Kuan, Y. F. Huang, C. C. Chang and S. L. Lo, Catalytic pyrolysis of sugarcane bagasse by using microwave heating, Bioresour. Technol., 2013, 146, 324–329 CrossRef CAS PubMed.
- W. Q. Liu, N. W. L. Low, B. Feng, G. X. Wang and J. C. D. da Costa, Calcium precursors for the production of CaO sorbents for multicycle CO2 capture, Environ. Sci. Technol., 2010, 44, 841–847 CrossRef CAS PubMed.
- E. Biagini, F. Barontini and L. Tognotti, Devolatilization of biomass fuels and biomass components studied by TG/FTIR technique, Ind. Eng. Chem. Res., 2006, 45, 4486–4493 CrossRef CAS.
- D. J. Nowakowski, J. M. Jones, R. M. D. Brydson and A. B. Ross, Potassium catalysis in the pyrolysis behavior of short rotation willow coppice, Fuel, 2007, 86, 2389–2402 CrossRef CAS PubMed.
- Y. Ohtsuka and A. Tomita, Calcium catalyzed steam gasification of Yallourn brown coal, Fuel, 1986, 651653–651657 Search PubMed.
- L. Yang, H. B. Yu, S. Q. Wang, H. W. Wang and Q. B. Zhou, Carbon Dioxide Captured from Flue Gas by Modified Ca-based Sorbents in Fixed-bed Reactor at High Temperature, Chin. J. Chem. Eng., 2013, 21, 199–204 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |