Controllable functionalized carbon fabric for high-performance all-carbon-based supercapacitors†
Abstract
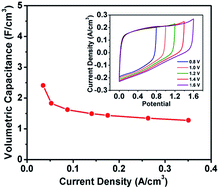
Research is currently being carried out to develop high-performance solid-state supercapacitors (SCs) with high energy density. One effective method to improve the energy density of the solid-state SC devices is enlarging their operating voltage. Organic electrolytes and asymmetric structure have been employed in supercapacitors to increase their operating voltage. However, they usually suffer from high cost, high toxicity, poor ionic conductivity and complicated manufacturing processes. Herein, a simple oxidation and annealing method is introduced to fabricate functionalized carbon fabric (FCF) electrodes. The as-prepared FCF electrode exhibits a high areal capacitance of 155.8 mF cm−2 and 143.4 mF cm−2 at negative potential and positive potential, respectively. Furthermore, a high-performance and flexible solid-state supercapacitor with high operating voltage (1.6 V) is successfully developed using functionalized carbon fabric as both positive and negative electrode. The FCF-based device has a high capacitance of 134.8 F cm−2 (or 2.41 F cm−3). The maximum volumetric energy density and volumetric power density are 0.83 mW h cm−3 and 1.58 W cm−3, respectively, which are higher than most previously reported CF-based SCs. The proposed FCF electrodes demonstrate exciting possibilities of developing high-performance solid-state supercapacitors with high operating voltage for efficient energy storage.

 Please wait while we load your content...
Please wait while we load your content...