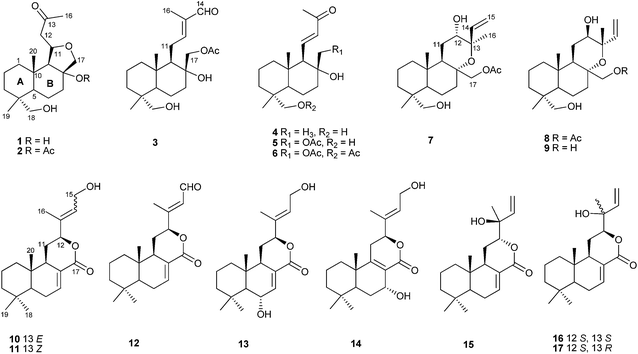
Labdane-type diterpenoids from Croton laevigatus†
Hong-Li Huang,
Feng-Ming Qi,
Ji-Cheng Yuan,
Cai-Gui Zhao,
Jing-Wei Yang,
Fu-Hu Fang,
Quan-Xiang Wu,
Kun Gao* and
Cheng-Shan Yuan*
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: yuancs@lzu.edu.cn; Fax: +86 0931 8915557; Tel: +86 0931 8912500
First published on 11th August 2014
Abstract
Sixteen new labdane-type diterpenoids (1–9, 11–17) were isolated from the twigs and leaves of Croton laevigatus, along with 15-hydroxylabda-7,13(E)-diene-17,12-olide (10), which is reported here as a natural product for the first time. Their structures were elucidated on the basis of extensive spectroscopic data interpretation, including UV, IR, NMR, and MS, and comparison with literature data. The structures of compounds 7, 8, 10, 14, 15, 16, and 17 were further confirmed by single-crystal X-ray diffraction analysis. The absolute configurations of 10–17 were determined by the CD exciton chirality method and supported by the single-crystal X-ray diffraction analysis of 10, 14, and 15. Crotonlaevins A (1) and B (2) are the reported first labda-type diterpenoids with a dodecahydronaphtho [1,2-c] furan moiety, and compounds 10–17 are the first reported labda-17,12-olide derivatives isolated from nature.
Introduction
Plants of the genus Croton (Euphorbiaceae) are rich sources of structurally diverse diterpenoids, which have brine shrimp lethality,1 antitumor,2 antimycobacterial,3 antifungal,4 and anti-inflammatory5 activities. Within this genus, 21 species mainly grow in the southern Provinces of China,6 and some of them have been used in traditional Chinese medicine to treat dysmenorrhea, gastric ulcers, malaria, gastric cancer, and dysentery.7–11 Croton laevigatus Vahl. is an arbor mainly distributed in the Yunnan, Guangdong and Hainan provinces of China. The twigs and leaves of Croton laevigatus, locally known as Bao-Long in Chinese, is a traditional Chinese medicine used for the treatment of bone fracture, malaria, and stomach ache.12 In prior reports, six cembranoids and a neocrotocembraneic acid were isolated from this species by Zou's group, among which laevigatlactone B exhibited modest cytotoxicity against HeLa cells.8 Moreover, two diterpenoids with an unprecedented backbone,11 and clerodane diterpenoids have also been discovered from this plant.13 As a continuation of the work on structurally interesting and biologically significant secondary metabolites of plant origin, we collected twigs and leaves of C. laevigatus from Yunnan Province and investigated the secondary metabolites of the EtOH extract. As a result, 16 new labdane-type diterpenoids (1–9, 11–17) and an additional new natural product (10) were obtained (Fig. 1). Herein, the isolation and structure elucidation of these compounds are reported.Results and discussion
Compound 1 was obtained as a colorless oil. Its molecular formula was established to be C18H30O4 from the [M + Na]+ peak at m/z 333.2043 (calcd 333.2036) in the positive HRESIMS, indicating four degrees of unsaturation. The IR absorptions suggested the presence of hydroxyl (3398 cm−1) and carbonyl (1709 cm−1) functionalities. Its 1H NMR spectroscopic data (Table 1) showed three tertiary methyls signals at δH 0.79, 0.96, and 2.18; two oxymethylene signals at δH 3.09 and 3.43 (d, J = 11.2 Hz, H2-18) as well as 3.68 and 3.76 (d, J = 9.6 Hz, H2-17); and one oxymethine signal at δH 4.43 (td, J = 5.2, 2.4 Hz, H-11). The 13C NMR spectroscopic data (Table 2) indicated 18 carbon signals, including two quaternary carbon signals at δC 35.9 and 37.5, two oxymethylene carbon signals at δC 71.5 and 78.5, one oxymethine carbon signal at δC 77.3, one oxygenated quaternary carbon signal at δC 80.1, and one ketone carbon signal at δC 208.1. The above observations indicated that 1 may be a nor-labdane-type diterpene, which was confirmed by the following analysis. The 1H–1H COSY experiment showed connectivity of three moieties: I: 1CH2–2CH2–3CH2; II: 5CH–6CH2–7CH2; and III: 9CH–11CH(O)–12CH2. The HMBC correlations from CH3-19 (δH 0.79, 3H, s) to C-3 (δC 35.1), C-5 (δC 44.4), and C-18 (δC 15.7); from H2-18 (δH 3.09 and 3.43, each 1H, d, J = 11.2 Hz) to C-3, C-5, and C-19 (δC 17.7); and from H2-2 (δH 1.50 and 1.60, each 1H, m) to C-4 (δC 37.5) indicated that C-4 connected units I and II together. The HMBC correlations from CH3-20 (δH 0.96, 3H, s) to C-1 (δC 41.4), C-5, and C-9 (δC 66.0) and from H2-1b (δH 0.96, 1H, m) to C-9 suggested that C-10 (δC 35.9) connected units I and III together. Furthermore, the HMBC correlations from H-9 (δH 1.57, 1H, d, J = 2.0 Hz) to C-17 (δC 78.5), from H-11 (δH 4.43, 1H, td, J = 5.2, 2.4 Hz) and H2-6 (δH 1.32 and 1.60, each 1H, m) to C-8 (δC 80.1), and from H2-17 (δH 3.68 and 3.76, each 1H, d, J = 9.6 Hz) to C-7 (δC 33.3), C-9, and C-11 (δC 77.3) implied that C-8 connected units II and III together. The above spectra delineated some structural fragments corresponding to AB rings of labdane-type diterpene. Moreover, the established molecular formula suggested the presence of an excess of epoxy group functionality. Considering the HMBC correlations from H2-17 to C-11, this epoxy group was positioned between C-11 and C-17, forming a tetrahydrofuran ring. The HMBC correlations from H3-16 (δH 2.18, 3H, s) to C-12 (δC 50.9) and C-13 (δC 208.1) implied that an acetyl group bonded to C-12. The relative configuration of 1 was determined by the NOE difference spectra and ROESY experiments. In the ROESY and NOE difference spectra, the presence of correlations of H-11, H2-17 and H3-19 to H3-20; H2-18 to H-5, and H-9 to H-5 and H2-12 demonstrated that H-5, 8-OH, H-9 and H2-18 are α-oriented whereas H-11, H2-17, H3-19 and H3-20 are β-oriented. Consequently, the structure of 1 was deduced as crotonlaevin A. Compound 2 was isolated as a white, amorphous powder. It was assigned to have a molecular formula of C20H32O5 by HRESIMS, which gave a pseudo-molecular ion at m/z 370.2594 [M + NH4]+ (calcd 370.2588). Spectroscopic data (Experimental section) of 2 were closely similar in structure to those of 1. The major difference was the presence of additional acetyl group signals (δH 2.02, 3H, s; δC 22.3, 170.0). Correspondingly, the oxygenated quaternary carbon signal C-8 was shifted downfield to δC 90.3 in 2, which indicated that 2 was an acetylated derivative of 1 at C-17; moreover, the 2D NMR experiments confirmed the proved structure. Therefore, the structure of 2 was identified as crotonlaevin B.| 1a | 3a | 4a | 7b | 12a | 15a | |
|---|---|---|---|---|---|---|
| Position | δH mult (J in Hz) | δH mult (J in Hz) | δH mult (J in Hz) | δH mult (J in Hz) | δH mult (J in Hz) | δH mult (J in Hz) |
| a Data were measured in CDCl3.b Data were measured in CD3OD.1H assignments aided by DEPT,1H–1H COSY, HSQC, HMBC and ROESY experiments. (400 MHz). | ||||||
| 1 | 0.96 m | 0.94 m | 0.89 td (13.2, 3.6) | 0.96 td (13.2, 3.6) | 1.09 td (12.8, 4.4) | 0.96 td (12.8, 4.0) |
| 1.67 m | 1.53 m | 1.36 m | 1.63 m | 1.77 d (12.8) | 1.72 m | |
| 2 | 1.50 m | 1.54 m | 1.48 m | 1.55 m | 1.56 m | 1.50 m |
| 1.60 m | 1.58 m | 1.60 m | 1.70 m | 1.61 m | 1.56 td (13.6, 3.2) | |
| 3 | 1.28 m | 1.27 m | 1.23 m | 1.25 m | 1.21 td (13.2, 4.4) | 1.19 td (12.8, 4.0) |
| 1.45 m | 1.50 m | 1.45 m | 1.51 m | 1.51 m | 1.46 m | |
| 5 | 1.43 m | 1.44 m | 1.34 m | 1.43 m | 1.33 dd (12.8, 4.8) | 1.34 dd (5.2, 4.8) |
| 6 | 1.32 m | 1.62 m | 1.31 m | 1.29 m | 2.12 m | 2.08 m |
| 1.60 m | 1.64 m | 1.62 m | 1.59 m | 2.41 m | 2.35 m | |
| 7 | 1.69 m | 1.39 m | 1.75 m | 1.32 m | 7.39 m | 7.03 m |
| 2.05 m | 2.09 dt (13.2, 3.2) | 1.89 m | 1.99 m | |||
| 9 | 1.57 d (2.0) | 1.72 t (5.2) | 2.00 d (10.8) | 2.01 m | 2.32 m | 2.47 m |
| 11 | 4.43 td (5.2, 2.4) | 2.35 m | 6.83 dd (15.6, 15.6) | 1.67 m | 1.48 m | 1.77 m |
| 2.66 dt (16.4, 4.4) | 1.83 td (13.6, 2.8) | 1.95 m | 1.88 m | |||
| 12 | 2.78 dd (16.0, 5.2) | 6.61 t (6.8) | 6.18 d (15.6) | 4.09 t (3.5) | 4.75 d (11.2) | 3.98 dd (4.0, 4.0) |
| 3.10 dd (16.0, 5.2) | ||||||
| 14 | 9.38 s | 6.10 dd (18.2, 18.2) | 6.13 d (7.6) | 5.87 dd (17.2, 17.2) | ||
| 15 | 4.98 d (11.5) | 10.08 d (7.6) | 5.21 dd (10.8, 0.8) | |||
| 5.08 d (18.2) | 5.40 dd (17.2, 0.8) | |||||
| 16 | 2.18 s | 1.77 s | 2.27 s | 1.19 s | 2.22 s | 1.40 s |
| 17 | 3.68 d (9.6) | 3.99 dd (11.2, 1.2) | 1.26 s | 4.28 d (12.4) | ||
| 3.76 d (9.6) | 4.38 d (11.2) | 4.44 d (12.4) | ||||
| 18 | 3.09 d (11.2) | 3.12 d (10.8) | 3.09 d (10.8) | 3.00 d (11.1) | 0.91 s | 0.88 s |
| 3.43 d (11.2) | 3.43 d (10.8) | 3.42 d (10.8) | 3.36 d (11.1) | |||
| 19 | 0.79 s | 0.76 s | 0.76 s | 0.73 s | 0.93 s | 0.90 s |
| 20 | 0.96 s | 0.93 s | 1.02 s | 0.85 s | 0.77 s | 0.75 s |
| 17-OAc | 2.13 s | 2.10 s | ||||
| carbon | 1a | 3a | 4a | 7b | 8a | 11a | 12a | 13c | 14d | 15a | 16a | 17a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Data were measured in CDCl3.b Data were measured in CD3OD.c Data were measured in acetone-D6.d Data were measured in C5D5N. The assignments were based on DEPT, HSQC, and HMBC experiments. (100 MHz). | ||||||||||||
| 1 | 41.4, t | 39.6, t | 40.5, t | 39.9, t | 38.7, t | 38.6, t | 38.7, t | 39.5, t | 36.9, t | 38.1, t | 38.6, t | 38.6, t |
| 2 | 17.6, t | 17.7, t | 17.6, t | 19.2, t | 17.9, t | 18.5, t | 18.5, t | 19.4, t | 19.5, t | 18.4, t | 18.5, t | 18.5, t |
| 3 | 35.1, t | 34.9, t | 35.0, t | 36.5, t | 35.1, t | 41.9, t | 41.8, t | 44.3, t | 41.8, t | 41.8, t | 41.9, t | 41.8, t |
| 4 | 37.5, s | 37.7, s | 48.0, s | 38.8, s | 37.6, s | 32.8, s | 32.8, s | 33.9, s | 33.7, s | 32.8, s | 32.8, s | 32.8, s |
| 5 | 44.4, d | 49.1, d | 48.4, d | 51.1, d | 49.5, d | 48.8, d | 48.8, d | 56.2, d | 45.1, d | 49.1, d | 48.9, d | 48.8, d |
| 6 | 18.8, t | 19.6, t | 19.8, t | 20.2, t | 19.3, t | 25.2, t | 25.2, t | 68.5, d | 29.0, t | 24.8, t | 25.2, t | 25.1, t |
| 7 | 33.3, t | 37.7, t | 42.7, t | 37.8, t | 37.1, t | 143.4, d | 144.2, d | 144.6, d | 62.3, d | 141.1, d | 143.4, d | 143.5, d |
| 8 | 80.1, s | 73.8, s | 72.3, s | 77.9, s | 75.0, s | 125.6, s | 125.2, s | 127.0, s | 126.3, s | 127.4, s | 125.9, s | 125.8, s |
| 9 | 66.0, d | 60.6, d | 65.8, d | 50.7, d | 56.6, d | 49.2, d | 49.1, d | 49.5, d | 162.4, s | 43.6, d | 48.6, d | 48.6, d |
| 10 | 35.9, s | 37.6, s | 35.2, s | 37.5, s | 36.7, s | 34.7, s | 34.8, s | 33.9, s | 40.0, s | 35.4, s | 34.8, s | 34.8, s |
| 11 | 77.3, d | 24.7, d | 144.7, d | 24.8, t | 24.0, t | 27.0, t | 27.5, t | 27.9, t | 27.3, t | 22.3, t | 22.6, t | 22.6, t |
| 12 | 50.9, t | 158.2, d | 69.3, d | 69.3, t | 74.8, d | 77.0, d | 81.8, d | 84.4, d | 81.2, d | 82.0, d | 84.6, d | 84.6, d |
| 13 | 208.1, s | 137.3, s | 197.8, s | 78.1, s | 77.7, s | 137.2, s | 157.5, s | 135.3, s | 134.1, s | 74.6, s | 74.0, s | 74.1, s |
| 14 | 195.4, s | 147.3, d | 145.2, d | 127.8, d | 126.6, d | 129.4, d | 131.0, d | 139.9, d | 140.6, d | 139.7, d | ||
| 15 | 112.5, t | 113.6, t | 58.1, t | 191.0, s | 58.8, t | 59.0, t | 114.5, t | 114.9, t | 114.9, t | |||
| 16 | 30.9, q | 9.3, q | 27.6, q | 27.6, q | 19.1, q | 18.3, q | 13.4, q | 12.1, q | 13.0, q | 25.6, q | 23.4, q | 24.2, q |
| 17 | 78.5, t | 65.7, t | 24.8, q | 63.9, t | 63.8, t | 165.8, s | 164.6, s | 165.5, s | 166.7, s | 168.3, s | 165.5, s | 165.7, s |
| 18 | 71.5, t | 71.8, t | 71.6, t | 22.7, q | 71.6, t | 32.9, q | 32.9, q | 36.8, q | 33.5, q | 32.7, q | 32.9, q | 32.9, q |
| 19 | 17.7, q | 17.3, q | 17.5, q | 17.7, q | 17.0, q | 21.4, q | 21.4, q | 22.6, q | 22.2, q | 21.4, q | 21.4, q | 21.4, q |
| 20 | 15.7, q | 16.4, q | 16.3, q | 17.5, q | 16.9, q | 13.4, q | 13.4, q | 15.0, q | 18.9, q | 12.9, q | 13.4, q | 13.4, q |
| 17-OAc | 171.1, s | 173.2, s | 171.8, s | |||||||||
| 20.9, q | 21.1, q | 21.2, q | ||||||||||
Compound 3 possessed a molecular formula of C21H34O5 with five degrees of unsaturation based on the [M + NH4]+ peak at m/z 384.2749 (calcd 384.2744) in the positive HRESIMS. The IR spectrum showed absorption bands for hydroxyl (3422 cm−1), ester carbonyl (1735 cm−1) and olefin (1676 cm−1) functionalities. The 1H and 13C NMR spectroscopic data (Tables 1 and 2) of 3 resembled those of 1 and 2, sharing the same AB rings, and also belongs to nor-labdane-type diterpene series. The structural feature was further corroborated by the 2D NMR experiments. A 1H–1H COSY experiment showed connectivity of a moiety: 9CH–11CH2–12CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 13C–16CH3. The HMBC spectrum showed correlations of H-9 (δH 1.72, 1H, t, J = 5.2 Hz) to C-17 (δC 65.7) and C-20 (δC 16.4) and CH3-20 (δH 0.93, s, 3H) to C-9 (δC 60.6), which revealed that the moiety was connected to the B ring at C-9. The 1H NMR spectrum displayed one aldehyde group signal at δH 9.38 (s, CHO-14). The HMBC experiment established correlations from CHO-14 to C-12 (δC 158.2), C-13 (δC 137.3) and C-16 (δC 9.3), which indicated the presence of an aldehyde group at C-13. Moreover, the 1H and 13C NMR spectroscopic data also showed an extra acetyl group signal at δH 2.13 (s, 3H; δC 20.9, 171.1). The HMBC experiment demonstrated correlations of H2-17 (δH 3.99 dd, J = 11.2, 1.2 Hz; 4.38, d, J = 11.2 Hz) to C-21 (δC 171.1), which indicated the acetoxyl group attached to C-17 in 3. The NOESY spectrum showed correlations of H-9 to H-5 and H2-17 to CH3-20, which indicated that the H2-17 was β-oriented, and the 8-OH and H-9 were α-oriented. The NOESY correlations of H2-11 to CH3-16 and CHO-14 to H-12 indicated that the olefinic bond at C-12 was E-type. Finally, the structure of 3 was assigned as crotonlaevin C.
13C–16CH3. The HMBC spectrum showed correlations of H-9 (δH 1.72, 1H, t, J = 5.2 Hz) to C-17 (δC 65.7) and C-20 (δC 16.4) and CH3-20 (δH 0.93, s, 3H) to C-9 (δC 60.6), which revealed that the moiety was connected to the B ring at C-9. The 1H NMR spectrum displayed one aldehyde group signal at δH 9.38 (s, CHO-14). The HMBC experiment established correlations from CHO-14 to C-12 (δC 158.2), C-13 (δC 137.3) and C-16 (δC 9.3), which indicated the presence of an aldehyde group at C-13. Moreover, the 1H and 13C NMR spectroscopic data also showed an extra acetyl group signal at δH 2.13 (s, 3H; δC 20.9, 171.1). The HMBC experiment demonstrated correlations of H2-17 (δH 3.99 dd, J = 11.2, 1.2 Hz; 4.38, d, J = 11.2 Hz) to C-21 (δC 171.1), which indicated the acetoxyl group attached to C-17 in 3. The NOESY spectrum showed correlations of H-9 to H-5 and H2-17 to CH3-20, which indicated that the H2-17 was β-oriented, and the 8-OH and H-9 were α-oriented. The NOESY correlations of H2-11 to CH3-16 and CHO-14 to H-12 indicated that the olefinic bond at C-12 was E-type. Finally, the structure of 3 was assigned as crotonlaevin C.
Compound 4 had the molecular formula of C18H30O3 with four degrees of unsaturation as determined by HRESIMS at m/z 312.2526 [M + NH4]+ (calcd 312.2533). The IR absorptions revealed the presence of hydroxyl (3372 cm−1), carbonyl (1693 cm−1) and olefin (1658 cm−1) functionalities. Analysis of the NMR spectroscopic data (Tables 1 and 2) of 4 established that 4 shared a closely similar structure with (11E)-14,15-bisnor-8α-hydroxy-11-labden-13-one.15,16 The major difference was that the methyl group CH3-18 signals (δH 1.02, 3H, s; δC 34.3) in the known compound were replaced by oxymethylene group CH2-18 signals (δH 3.09 and 3.42, each 1H, d, J = 10.8 Hz; δC 71.6) in 4. The above observations identified 4 as an 18-oxygenated derivative of the known compound. Considering the established molecular formula and the diagnostic chemical shift value of C-18, a hydroxyl group should be bonded to C-18. The relative configuration and the conformation of the olefinic system Δ11,12 provided by the NOE difference spectra and ROESY experiment were the same as those of (11E)-14,15-bisnor-8α-hydroxy-11-labden-13-one. In particular, the NOE enhancements of H-11 to H3-16 and H2-17 to H3-20 indicated the β-orientation of H-17 and the E-type of Δ.11,12 Thus, the structure of 4 was determined as crotonlaevin D.
Compound 5 was isolated as a colorless oil. It was assigned to have a molecular formula of C20H32O5 by HRESIMS at m/z 370.2579 [M + NH4]+ (calcd 370.2588). Comparison of its 1H and 13C NMR spectroscopic data (Experimental section) with those of 4 indicated that the two compounds were structurally similar. The major difference was that the C-17 signal at δC 24.8 in 4 was replaced with an oxymethylene carbon signal at δC 67.9 in 5. Correspondingly, oxygenated methylene proton signals at δH 4.17 and 4.29 (each 1H, d, J = 12.0 Hz) in 5 were observed in the 1H NMR spectrum. These observations suggested that the Me-17 in 4 was oxidized to an oxymethylene group in 5. The 1H and 13C NMR spectra also showed additional acetyl group signals at δH 2.09 (3H, s) and δC 20.9, 171.2, indicating that the acetoxyl group was attached to C-17. This conclusion was confirmed by the HMBC correlation from H2-17 to C-21. The above information indicated that 5 was a 17-acetylated derivative of 4, crotonlaevin E.
Compound 6 was obtained as a white, amorphous powder. Its molecular weight was determined by the peak at m/z 412.2690 in the positive HRESIMS ([M + NH4]+, calcd 412.2694), corresponding to a molecular formula of C22H34O6, which required six degrees of unsaturation. Compared with 5, the 1H and 13C NMR spectroscopic data (Experimental section) of 6 showed extra acetyl group signals at δH 2.10 (3H, s) and δC 21.0, 171.1, indicating that it was an acetylated derivative of 5. In addition, the chemical shifts of CH2-18 changed from δH 3.09 and 3.41 (each 1H, d, J = 10.8 Hz; δC 71.5) in 5 to δH 3.62 and 3.88 (each 1H, d, J = 10.8 Hz; δC 72.3) in 6, which were interpreted to represent an acetoxyl group at C-18. This conclusion was supported by the HMBC correlations from H2-18 to the carbonyl carbon signal of the acetyl group. Therefore, 6 was a 18-acetylated derivative of 5, crotonlaevin F.
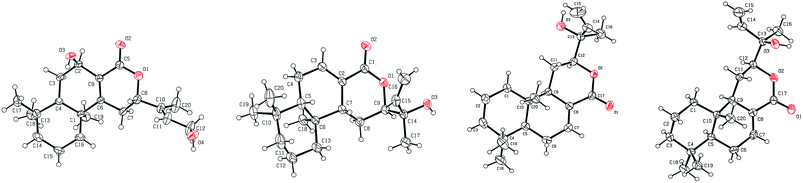
Compound 7 was obtained as colorless crystals (mixed solvents: petroleum ether/MeOH/CH3CN), with a molecular formula of C22H36O5 as determined by the [M + H]+ peak at m/z 381.2638 (calcd 381.2636) in the positive HRESIMS, indicating five degrees of unsaturation. The IR spectrum showed absorption bands due to hydroxyl (3328 cm−1) and ester carbonyl (1735 cm−1) functionalities. The 1H and 13C NMR spectroscopic data (Tables 1 and 2) of 7 resembled those of 18-hydroxy-13-epi-manoyl oxide.17 The major difference was that the methyl H3-17 signals and methylene group H2-12 signals in the known compound were replaced by the oxymethylene group H2-17 signals (δH 3.00 and 3.36, d, J = 11.1 Hz, each 1H; 4.28 and 4.44, d, J = 12.4 Hz, each 1H; δC 63.9) and the oxymethine group H-12 signals (δH 4.09, t, J = 3.5 Hz, 1H; δC 69.3) in 7. These observations suggested that 7 was a 12- and 17-oxygenated derivative of the known compound. Considering the established formula, a hydroxyl group should be present and bonded to C-12. Moreover, extra acetyl group signals (δH 2.10, s, 3H; δC 21.1, 173.2) were observed in the 1H and 13C NMR spectra. H2-17 showed HMBC correlations to C-7 (δC 37.8), C-8 (δC 77.9), and C-21 (δC 173.2, s), indicating that the acetoxyl group was located at C-17. The NOE difference spectra and ROESY experiments provided the correlations of H-9 to H-5, H-12 to H-14 and H3-22, and H2-17 to H-14 and H3-20, indicating that H-12, H-14 and H2-17 were β-oriented, whereas H-9 and 12-OH were α-oriented. This conclusion was in accordance with the known compound. However, there were no correlations of H3-16 to H-9 or OH-12 observed in NOE difference spectrum and NOESY experiments. To confirm the structure and the stereochemistry, 7 was crystallized in petroleum ether/MeOH/CH3CN to obtain a crystal of the monoclinic space group, which was analyzed by X-ray crystallography. Single-crystal X-ray diffraction analysis (Fig. 2A) showed that CH3-16 was α-oriented. The absolute configuration was determined by the measurement of the Flack parameter, which is calculated during the structural refinement.18,19 The final refinement on the Cu Kα data of the crystal of 7 resulted in a Flack parameter of 0.03 (6),18 allowing an unambiguous assignment of the absolute structure as shown. The seven chiral centers, C-4, C-5, C-8, C-9, C-10, C-12, and C-13, were thus determined to be R, R, R, R, S, S, and R, respectively. Thus, 7 was determined to be a manoyl oxide derivative and named crotonlaevin G.
HRESIMS, IR, and NMR spectroscopic data (Experimental section and Table 2) indicated that 8 has the same planar structure as 7, and the difference may be in the configuration. This conclusion was supported by NOE difference experiments. The NOE difference spectra showed NOE enhancements of H-9 to H-5 and H-12 as well as H3-16 to H2-17, which indicated that the OH-12 and H3-16 were β-oriented and H-12 and H-14 were α-oriented. The structure was confirmed by single-crystal X-ray diffraction analysis (Fig. 2B). Considering their closely similar NMR patterns and their coexistence in the same plant, compounds 7 and 8 may possess the same absolute configurations at corresponding stereocenters, except for C-12 and C-13. Finally, the structure of 8 was elucidated as crotonlaevin H.
Compound 9, isolated as a colorless oil, possessed a molecular formula of C20H34O4 as established by HRESIMS ([M + NH4]+ at m/z 356.2793). The IR spectrum showed absorption bands due to hydroxyl (3414 cm−1) and olefin (1709 cm−1) functionalities. 9 shared similar 1H and 13C NMR patterns (Experimental section) to those of 8. However, the acetyl group signal in 9 was missing. Hence, the structure of 9 was a 17-deacetylated derivative of 8 and was assigned as crotonlaevin I.
Compound 10, a white amorphous powder, presented a molecular formula of C20H30O3 as determined by HRESIMS at m/z 341.2083 [M + Na]+ (calcd 341.2087) with six degrees of unsaturation. The 1H and 13C NMR spectroscopic data (Experimental section) of 10 were in good agreement with literature data of 15-hydroxylabda-7,13(E)-diene-17,12-olide,20 the semisynthesis of which has been already described. Here 10 was isolated as a new natural product, and its relative configuration was determined by NOE difference experiments. The NOE enhancements of H-9 to H-5 and H-12, H-12 to H-14, and H2-15 to H3-16 indicated that H-9 and H-12 were α-oriented and the olefinic bond at C-13 was E-type, which supported the previously reported relative configuration.
The absolute configuration of 10 was determined by applying the CD exciton chirality method.21 The UV spectrum of 10 showed a strong absorption at λmax 229 nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε 6.87) attributable to the α,β-unsaturated lactone (π–π*, Woodward Rule: 229 nm).22 Corresponding to this UV maximum, the CD spectrum of 10 (Fig. 3B) showed a positive Cotton effect at λmax 234 nm (Δε + 0.21) and a negative Cotton effect at λmax 214 nm (Δε − 0.0095) due to the transition interaction between two different chromophores of the α,β-unsaturated lactone and Δ13,14 double bond. The above information demonstrated a positive chirality for 10 and the two aforementioned chromophores should be oriented clockwise in space23 (Fig. 3B). Thus, the absolute configurations of 10 were established as 5S, 9R, 10S and 12S.
ε 6.87) attributable to the α,β-unsaturated lactone (π–π*, Woodward Rule: 229 nm).22 Corresponding to this UV maximum, the CD spectrum of 10 (Fig. 3B) showed a positive Cotton effect at λmax 234 nm (Δε + 0.21) and a negative Cotton effect at λmax 214 nm (Δε − 0.0095) due to the transition interaction between two different chromophores of the α,β-unsaturated lactone and Δ13,14 double bond. The above information demonstrated a positive chirality for 10 and the two aforementioned chromophores should be oriented clockwise in space23 (Fig. 3B). Thus, the absolute configurations of 10 were established as 5S, 9R, 10S and 12S.
 | ||
| Fig. 3 X-ray crystal structures of compound 10a. CD and UV spectra of compounds 10–17 (in MeOH). The arrow denotes the electronic transition dipole of the chromophores for compounds 10 and 14. | ||
To confirm the structure and the CD exciton chirality rule used to determine the absolute configuration, 10 was esterified with p-bromobenzoyl chloride, and the product 10a was crystallized in petroleum ether/EtOAc/MeOH to afford a crystal of the monoclinic space group P2(1), which was analyzed by X-ray crystallography (Fig. 3A). A bromine atom in the single-crystal determined the absolute configuration of 10 on the basis of Flack's method with Flack's parameter determined to be 0.008 (14).18 Single-crystal X-ray analysis confirmed the structure of 10.
Compound 11 gave a molecular formula of C20H30O3, as determined by the observed pseudo-molecular ion at 319.2267 [M + H]+ (calcd 319.2268) in the HRESIMS. In the 1H and 13C NMR spectra (Experimental section and Table 2), 11 showed similar signals to those observed for 10, except for changes at CH-12 (δH 5.16, 1H, dd, J = 11.2, 2.0 Hz; δC 77.0), C-13 (δC 137.2), CH-14 (δH 5.61, 1H, t, J = 6.8 Hz; δC 127.8), and CH3-16 (δH 1.80, 3H, s; δC 18.3). These data suggested that 10 and 11 were a pair of cis-trans isomers at Δ,13,14 and the olefinic bond in 11 at C-13 was Z-type. This inference was supported by the NOE enhancements of H-12 to H2-15 and H-14 to H3-16. The CD spectrum of 11 (Fig. 3B) also showed a split Cotton effect (λmax 235 nm Δε + 0.078, λmax 215 nm Δε − 0.079), which arose from the exciton coupling of the chromophores of the α,β-unsaturated lactone and Δ13,14 double bond with positive chirality. These split CD signals suggested the absolute configurations of 11 were 5S, 9R, 10S and 12S. Accordingly, the structure of 11 was established to be crotonlaevin J.
Compound 12, obtained as an amorphous powder, was shown to have a molecular formula of C20H28O3 on the basis of the HRESIMS at m/z 317.2112 [M + H]+ (calcd 317.2111). The UV spectrum showed absorption at λmax 234 nm due to an α,β-unsaturated lactone, while the IR spectrum showed absorption bands attributable to ester carbonyl (1711 cm−1) and olefin (1674 and 1638 cm−1) functionalities. A combined analysis of 1H and 13C NMR (Tables 1 and 2), HSQC, and HMBC spectra of 12 indicated that it was a closely related analogue of 10 sharing the same ABC rings and Δ.13,14 The only structural difference was the aldehyde group (δH 10.08, d, J = 7.6 Hz; δC 191.0) in 12 instead of the oxymethylene group (δH 4.17, t, J = 3.0 Hz; δC 58.7) in 10. This conclusion was further confirmed by the HMBC observations, in which the key correlations were from CHO-15 to C-13 (δC 157.5) and C-16 (δC 13.4). The relative configuration of 12 supported by the NOE difference experiment was in accordance with that of 10. The CD spectra of 10 and 12 (Fig. 3B) showed a similar CD split pattern. Based on a biogenetic analogy and comparisons with molecular models, the absolute configurations of 12 were established as 5S, 9R, 10S and 12S. Consequently, the structure of 12 was identified as crotonlaevin K.
Compound 13 was isolated as a white, amorphous powder. Its positive HRESIMS gave a [M + Na]+ peak at m/z 357.2025 (calcd 357.2034), consistent with a molecular formula of C20H30O4, which required six degrees of unsaturation. The UV spectrum showed the characteristic absorption maxima of α,β-unsaturated lactone at λmax 226 nm. The IR spectrum revealed the presence of hydroxyl (3385 cm−1), ester carbonyl (1705 cm−1) and olefin (1647 cm−1) functionalities. The 1H and 13C NMR spectroscopic data (Experimental section and Table 2) proved that compounds 13 and 10 were similar in structure, with the only difference being the presence of an excess of oxymethine signals for CH-6 (δH 4.35 dt, J = 10.4, 2.8 Hz; δC 68.5) in 13. Considering the established formula, a hydroxyl group should be present and bonded to C-6 based on the HMBC correlations from H-6 to C-4 (δC 33.9) and C-8 (δC 127.0). The coupling constant and the NOE enhancements of H-6 to H3-19 and H3-20 indicated the β-orientation of H-6 and the α-orientation of HO-6. With a similar CD spectrum (Fig. 3B) to that of 10, the absolute configurations of 13 were determined to be 5S, 6S, 9R, 10R and 12S. 13 was assigned as crotonlaevin L.
Compound 14 showed the molecular formula of C20H30O4, obtained from the [M + Na]+ peak at m/z 357.2034 (calcd 357.2036) in the positive HRESIMS. The IR spectrum exhibited absorption bands due to hydroxyl (3381, 3296 cm−1), conjugated carbonyl (1683 cm−1) and olefin (1625 cm−1) functionalities. The 1H and 13C NMR spectroscopic data of 14 (Experimental section and Table 2) were similar to those of 13, except for changes at CH2-6 (δH 1.68, 1H, dd, J = 13.6, 4.4 Hz, δH 2.11, 1H, d, J = 13.6 Hz; δC 29.0), CH-7 (δH 5.25, 1H, d, J = 4.0 Hz; δC 62.3), C-8 (δC 126.3), and C-9 (δC 162.4). These data suggested that a hydroxyl group should be bonded to C-7 and a double bond was at C-8. This conclusion was confirmed by the HMBC correlations of H-7 to C-5 (δC 45.1) as well as C-9 and C-17 (δC 166.7). In the NOE difference spectra and ROESY experiment, the presence of the correlations between H-12 and H-14 and between H2-15 and H3-16 demonstrated the olefinic bond at C-13 to be E-type. Unfortunately, the NMR spectra could not give useful information to determine the relative configuration of H-7 and H-12. A single-crystal X-ray diffraction analysis (Fig. 4A) of 14 showed that H-5, OH-7, H-12, and H3-18 were α-oriented, whereas H-7, H3-19, and H3-20 were β-oriented. The UV spectrum of 14 showed a strong absorption at λmax 221 nm, which resulted from the α,β-unsaturated lactone (π–π*, Woodward rule: 229 nm).22 Corresponding to this UV absorption, the CD spectrum (Fig. 3B) of 14 showed a negative Cotton effect centering at λmax 217 nm (Δε − 0.79), which indicated that the α,β-unsaturated lactone and the Δ13,14 double bond should be oriented counterclockwise in space and a negative chirality for 14.22 The above information demonstrated that the absolute configurations of 14 were 5S, 7R, 10S and 12S. The final refinement on the Cu Kα data of the crystal of 14 resulted in a Flack parameter of −0.03(6),18 which confirmed the structure. Thus, the structure of 14 was characterized as crotonlaevin M.
Compound 15 was obtained as colorless crystals (mixed solvents: petroleum ether/EtOAc/acetone) and had the molecular formula of C20H30O3 with six degrees of unsaturation as determined by HRESIMS at m/z 319.2257 [M + H]+ (calcd 319.2268). The UV absorption at λmax 223 nm was ascribed to the α,β-unsaturated lactone. The IR absorptions revealed the presence of hydroxyl (3427 cm−1), ester carbonyl (1707 cm−1) and olefin (1641 cm−1) functionalities. Detailed analysis of the NMR spectroscopic data (Tables 1 and 2) indicated that 15 was similar in structure to 10 bearing a α,β-unsaturated lactone. The 1H NMR spectrum showed one monosubstituted vinyl group signal for H-14 (δH 5.87, 1H, dd, J = 17.2, 17.2 Hz) and H2-15 (δH 5.21, 1H, dd, J = 10.8, 0.8 Hz; 5.40, dd, J = 17.2, 0.8 Hz). In the HMBC spectrum, H-14 showed correlations to C-12 (δC 82.0) and C-16 (δC 25.6), and H2-15 showed the correlation to C-13 (δC 74.6), indicating that the monosubstituted vinyl group was at C-13. The H3-16 (δH 1.40, 3H, s) showed HMBC correlations to C-12 and C-13, indicating that CH3-16 bonded to C-13. Considering the diagnostic chemical shift value of C-13 and the established molecular formula, a hydroxyl group was at C-13. Moreover, the 1H and 13C NMR data also showed one oxymethine signal for CH-12 (δH 3.98, dd, J = 4.0, 4.0 Hz; δC 82.0). The H-12 and H3-20 had the same orientation, as supported by the NOE enhancements of H-12 to H3-20. However, the relative configurations of OH-13 and CH3-16 could not be determined from the NOE difference spectra. A single-crystal X-ray diffraction analysis (Fig. 4B) showed that H-5, H-9, CH3-16, and CH3-18 were α-oriented, and H-12, OH-13, CH3-19, and CH3-20 were β-oriented. The similar CD spectra (Fig. 3) of 15 and 14 suggested that the absolute configurations of 15 were 5S, 9R, 10S, 12R and 13S (a negative Cotton effect centering at λmax 219 nm, Δε −0.44).22 The final refinement based on the Cu Kα data of the crystal of 15 resulted in a Flack parameter of 0.0(2),18 which confirmed the structure. Therefore, 15 was elucidated to be crotonlaevin N.
HRESIMS, UV, IR, CD, and NMR spectroscopic data (Experimental section and Table 2) of compounds 16 and 17 indicated that 16 shares a closely similar structural pattern as 17 and differs in the side chain. The changed proton and carbon signals of CH-14 and CH3-16 were observed in the 1H and 13C NMR spectra. Single-crystal X-ray diffraction analysis (Fig. 4C and D) showed that CH3-16 was α-oriented and OH-13 was β-oriented in 16 and that CH3-16 was β-oriented and OH-13 was α-oriented in 17, indicating that 16 and 17 were a pair of epimers at C-13. Compounds 16 and 17 showed similar CD spectra (Fig. 3B) to that of 10. Therefore, the absolute configurations of 16 were determined as 5S, 9R, 10S, 12S, and 13S, and 16 was named as crotonlaevin O. The absolute configurations of 17 were determined as 5S, 9R, 10S, 12S, and 13R, and 17 was named as crotonlaevin P.
Conclusions
In this study, sixteen new labdane-type diterpenoids (1–9, 11–17) were obtained. To the best of our knowledge, crotonlaevins A (1) and B (2) are the first labda-type diterpenoids with a dodecahydronaphtho[1,2-c] furan moiety. Although a few labda-17,12-olide derivatives have been semi-synthetized,20,24 compounds 10–17 are relatively uncommon, and they are the first labda-17,12-olide derivatives to be isolated from nature and reported in the literature.Experimental
General experimental procedures
Melting points were determined on an X-4 Digital Display micro-melting point apparatus, uncorrected. Optical rotations were measured on a Perkin-Elmer Model 341 polarimeter with a 1 dm cell. UV spectra were measured on a Shimadzu UV-260 spectrophotometer. CD spectra were recorded on an Olis DSM 1000 circular dichroism spectrophotometer. IR spectra were recorded on a Nicolet NEXUS 670 FT-IR spectrometer over the range of 400–4000 cm−1. NMR spectra were conducted on a Bruker AM-400BB (400 MHz) spectrometer (δ in ppm rel. to TMS, J in Hz). HRESIMS determinations were run on a Bruker APEX II mass spectrometer. Single-crystal X-ray diffractions were recorded on Bruker Smart Apex CCD diffractometer using graphite-monochromated Mo Kα or Cu Kα radiation. Sephadex LH-20 (Amersham Pharmacia Biotech), RP-C18 silica gel (150–200 mesh, Merck), and silica gel (200–300 mesh, Qingdao Marine Chemical Factory) were used for column chromatography (CC). Thin-layer chromatography (TLC): silica gel GF254 (10–40 μm; Qingdao Marine Chemical Factory); detection under UV light and visualized by spraying with 5% H2SO4 in C2H5OH (v/v), followed by heating. Analytical TLC was provided to follow the separation and check the purity of isolated compounds.Plant material
The twigs and leaves of Croton laevigatus Vahl. were collected from Yunnan Province, P. R. China, on October 2009, and authenticated by Prof. Guo-Da Tao of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences. A voucher specimen (no. 200910CL) was deposited in the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences.Extraction and isolation
The air-dried leaves and twigs of Croton laevigatus (8.0 kg) were crushed into a powder by a wood crusher, then extracted with EtOH (95%, v/v) three times (each for 3 hours) at 40 °C and concentrated in vacuo to give a crude extract (809 g). The crude extract was suspended in H2O (3.0 L) and then extracted with CHCl3 (3 × 2.0 L) and EtOAc (3 × 2.0 L), successively. The CHCl3-soluble and EtOAc-soluble fractions were combined due to similar constituents on TLC. The combined extract (244.7 g) was subjected to silica gel column chromatography (CC) (1.5 kg), eluting with a CHCl3–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–2
0–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, CH3OH) gradient system to afford fractions 1–6 under the aid of TLC examination. Fraction 1 was not further purified due to the presence of fatty materials as the main constituents on the basis of TLC analysis. Fraction 2 (43.4 g) was applied to silica gel eluting with petroleum ether–acetone (40
1, CH3OH) gradient system to afford fractions 1–6 under the aid of TLC examination. Fraction 1 was not further purified due to the presence of fatty materials as the main constituents on the basis of TLC analysis. Fraction 2 (43.4 g) was applied to silica gel eluting with petroleum ether–acetone (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide fractions 2a–2d. Fraction 2c (23.5 g) was repeatedly chromatographed on silica gel (petroleum ether–EtOAc 20
1) to provide fractions 2a–2d. Fraction 2c (23.5 g) was repeatedly chromatographed on silica gel (petroleum ether–EtOAc 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give fractions 2c1–2c3. Fraction 2c1 (2.6 g) was separated by CC over a silica gel (petroleum ether–acetone, 10
1) to give fractions 2c1–2c3. Fraction 2c1 (2.6 g) was separated by CC over a silica gel (petroleum ether–acetone, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0
1, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), MCI gel (MeOH–H2O, 0
1), MCI gel (MeOH–H2O, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), RP-18 silica gel (MeOH–H2O, 2
1), RP-18 silica gel (MeOH–H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and silica gel (petroleum ether–EtOAc, 9
1), and silica gel (petroleum ether–EtOAc, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 10 (13 mg) and 11 (16 mg). The fraction 2c2 (4.6 g) was chromatographed over a MCI gel (MeOH–H2O, 0
1) to give 10 (13 mg) and 11 (16 mg). The fraction 2c2 (4.6 g) was chromatographed over a MCI gel (MeOH–H2O, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), RP-18 silica gel (MeOH–H2O, 1
1), RP-18 silica gel (MeOH–H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1.5
1–1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and silica gel (300–400 mesh, petroleum ether–EtOAc, 10
1), and silica gel (300–400 mesh, petroleum ether–EtOAc, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 15 (35 mg), 16 (27 mg), and 17 (21 mg). Fraction 3 (51.1 g) was rechromatographed over a Sephadex LH-20 column (CH2Cl2–CH3OH 1
1) to afford 15 (35 mg), 16 (27 mg), and 17 (21 mg). Fraction 3 (51.1 g) was rechromatographed over a Sephadex LH-20 column (CH2Cl2–CH3OH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and silica gel (CHCl3–acetone 40
1) and silica gel (CHCl3–acetone 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0
1, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide fractions 3a–3f. Fraction 3b (6.7 g) was subjected to a column of MCI gel (MeOH–H2O, 0
1) to provide fractions 3a–3f. Fraction 3b (6.7 g) was subjected to a column of MCI gel (MeOH–H2O, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), RP-18 silica gel (MeOH–H2O, 1
1), RP-18 silica gel (MeOH–H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–2
2–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and silica gel CC (petroleum ether–EtOAc, 1
1), and silica gel CC (petroleum ether–EtOAc, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0
1, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield 6 (15 mg). A similar isolation procedure as used for fraction 3b was adopted for fraction 3d (8.1 g) to afford 2 (4 mg), 13 (8 mg) and 14 (16 mg). 5 (24 mg) was isolated from fraction 3e (3.4 g) after preparative TLC (petroleum ether–acetone, 1.5
1) to yield 6 (15 mg). A similar isolation procedure as used for fraction 3b was adopted for fraction 3d (8.1 g) to afford 2 (4 mg), 13 (8 mg) and 14 (16 mg). 5 (24 mg) was isolated from fraction 3e (3.4 g) after preparative TLC (petroleum ether–acetone, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Fraction 4 (11.3 g) was fractionated using silica gel (petroleum ether–acetone, 10
1). Fraction 4 (11.3 g) was fractionated using silica gel (petroleum ether–acetone, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0
1, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), then subjected to a column of RP-18 silica gel (MeOH–H2O, 1
1), then subjected to a column of RP-18 silica gel (MeOH–H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and further purified on Sephadex LH-20 (CH2Cl2–MeOH, 1
1), and further purified on Sephadex LH-20 (CH2Cl2–MeOH, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain 12 (21 mg). After CC over silica gel developed with CHCl3–acetone (10
1) to obtain 12 (21 mg). After CC over silica gel developed with CHCl3–acetone (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0
1, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), fraction 5 (93.4 g) afforded fractions 5a–5c. 3 (5 mg), 4 (18 mg) and 8 (8 mg) were obtained from fraction 5a (5.1 g) after repeated silica gel CC eluting with petroleum ether–acetone (3
1), fraction 5 (93.4 g) afforded fractions 5a–5c. 3 (5 mg), 4 (18 mg) and 8 (8 mg) were obtained from fraction 5a (5.1 g) after repeated silica gel CC eluting with petroleum ether–acetone (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0
1, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Fraction 5b (8.9 g) was chromatographed on silica gel (petroleum ether–acetone, 6
1). Fraction 5b (8.9 g) was chromatographed on silica gel (petroleum ether–acetone, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0
1, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), followed by RP-18 silica gel (MeOH–H2O, 1
1), followed by RP-18 silica gel (MeOH–H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) and silica gel (300–400 mesh, petroleum ether–acetone, 3
1.5) and silica gel (300–400 mesh, petroleum ether–acetone, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0
1, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), to give 7 (29 mg) and 9 (9 mg). Fraction 6 (11.2 g) was purified on a column of MCI gel (MeOH–H2O 1
1), to give 7 (29 mg) and 9 (9 mg). Fraction 6 (11.2 g) was purified on a column of MCI gel (MeOH–H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–2
2–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), RP-18 silica gel (MeOH–H2O, 1
1), RP-18 silica gel (MeOH–H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5), and silica gel (300–400 mesh, petroleum ether–EtOAc, 1
1.5), and silica gel (300–400 mesh, petroleum ether–EtOAc, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 0
3, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 1 (6 mg).
1) to afford 1 (6 mg).
Spectroscopic data of compounds 1–17
Crotonlaevin A (1): colorless oil; [α]26D −11.43 (c 0.35, in CHCl3); IR (KBr) νmax 3398, 2930, 2872, and 1709 cm−1; 1H NMR and 13C NMR data (CDCl3, see Tables 1 and 2); HRESIMS m/z 333.2043 [M + Na]+ (calcd for C18H30O4Na +, 333.2036).Crotonlaevin B (2): white amorphous powder; [α]26D −23.33 (c 0.30, in CHCl3); IR (KBr) νmax 3378, 2933, 2873, 1724, and 1700 cm−1; 1H NMR (CDCl3, 400 MHz) δH: 4.46 (1H, td, J = 5.2, 2.8 Hz, H-11), 4.40 (1H, d, J = 10.8 Hz, H2-17a), 3.75 (1H, d, J = 10.8 Hz, H2-17b), 3.40 (1H, d, J = 8.8 Hz, H2-18a), 3.14 (1H, d, J = 8.8 Hz, H2-18b), 2.92(1H, dd, J = 15.6, 8.4 Hz, H2-12a), 2.58 (1H, dd, J = 15.6, 8.4 Hz, H2-12b), 2.46 (1H, dt, J = 14.0, 5.2 Hz, H2-7a), 1.99 (1H, m, H2-7b), 2.19 (3H, s, H3-16), 2.02 (3H, s, H3-22), 1.86 (1H, d, J = 2.0 Hz, H-9), 1.69 (1H, m, H2-1a), 0.98 (1H, m, H2-1b), 1.60 (1H, m, H2-2a), 1.52 (1H, m, H2-2b), 1.60 (1H, m, H2-6a), 1.32 (1H, m, H2-6b), 1.50 (1H, m, H-5), 1.44 (1H, m, H2-3a), 1.32 (1H, m, H2-3b), 0.99 (3H, s, H3-20), 0.80 (3H, s, H3-19); 13C NMR (CDCl3, 100 MHz) δC: 207.1 (C-13, s), 170.0 (C-21, s), 90.3 (C-8, s), 76.4 (C-11, d), 74.6 (C-17, t), 71.7 (C-18, t), 63.6 (C-9, d), 50.7 (C-12, t), 43.5 (C-5, d), 41.5 (C-1, t), 37.4 (C-4, s), 35.9 (C-10, s), 35.1 (C-3, t), 29.0 (C-7, t), 30.9 (C-14, q), 22.3 (C-22 q), 18.5 (C-6, t), 17.6 (C-19, q), 17.5 (C-2, t), 15.9 (C-20, q); HRESIMS m/z 370.2594 [M + NH4]+ (calcd for C20H36O5N+, 370.2588).
Crotonlaevin C (3): colorless oil; [α]26D +12.15 (c 0.11, in CHCl3); IR (KBr) νmax 3422, 2926, 2869, 1735, and 1676 cm−1; 1H NMR and 13C NMR data (CDCl3, see Tables 1 and 2); HRESIMS m/z 384.2749 [M + NH4]+ (calcd for C21H38O5N+, 384.2744).
Crotonlaevin D (4): colorless oil; [α]26D +16.67 (c 0.24, in CHCl3); IR (KBr) νmax 3372, 2933, 2882, 2862, 1693, and 1658 cm−1; 1H NMR and 13C NMR data (CDCl3, see Tables 1 and 2); HRESIMS m/z 312.2526 [M + NH4]+ (calcd for C18H34O3N+, 312.2533).
Crotonlaevin E (5): colorless oil; [α]26D +11.11 (c 0.54, in CHCl3); IR (KBr) νmax 3443, 2929, 2874, 1736, and 1667 cm−1; 1H NMR (CDCl3, 400 MHz) δH: 6.84 (1H, dd, J = 15.6, 15.6 Hz, H-11), 6.21 (1H, d, J = 15.6 Hz, H-12), 4.29 (1H, d, J = 12.0 Hz, H2-17a), 4.17 (1H, d, J = 12.0 Hz, H2-17b), 3.41 (1H, d, J = 10.8 Hz, H2-18a), 3.09 (1H, d, J = 10.8 Hz, H2-18b), 2.25 (3H, s, H3-16), 2.15 (1H, m, H-9), 2.09 (3H, s, H3-22), 2.04 (1H, m, H2-7a), 1.46 (1H, m, H2-7b), 1.64 (1H, m, H2-6a), 1.28 (1H, m, H2-6b), 1.61 (1H, m, H2-2a), 1.49 (1H, m, H2-2b), 1.43 (1H, m, H2-3a), 1.24 (1H, m, H2-3b), 1.35 (1H, m, H-5), 1.32 (1H, m, H2-1a), 0.89 (1H, td, J = 12.4, 2.8 Hz, H2-1b), 1.04 (3H, s, H3-20), 0.75 (3H, s, H3-19); 13C NMR (CDCl3, 100 MHz) δC: 197.5 (C-13, s), 171.2 (C-21, s), 143.5 (C-11, d), 134.7 (C-12, d), 73.2 (C-8, s), 71.5 (C-18, t), 67.9 (C-17, t), 64.8 (C-9, d), 48.5 (C-5, d), 40.4 (C-1, t), 37.6 (C-4, s), 37.4 (C-10, s), 37.1 (C-7, t), 34.9 (C-3, t), 27.9 (C-16, q), 20.9 (C-22, q), 19.4 (C-6, t), 17.6 (C-2, t), 17.4 (C-19, q), 16.4 (C-20, q); HRESIMS m/z 312.2526 [M + NH4]+ (calcd for C18H34O3N+, 312.2533).
Crotonlaevin F (6): colorless oil; [α]26D +23.08 (c 0.39, in CHCl3); IR (KBr) νmax 3470, 2933, 2877, 1737, and 1670 cm−1; 1H NMR (CDCl3, 400 MHz) δH: 6.84 (1H, dd, J = 15.6, 15.6 Hz, H-11), 6.24 (1H, d, J = 15.6 Hz, H-12), 4.29 (1H, d, J = 11.6 Hz, H2-17a), 4.18 (1H, d, J = 11.6 Hz, H2-17b), 3.88 (1H, d, J = 10.8 Hz, H2-18a), 3.62 (1H, d, J = 10.8 Hz, H2-18b), 2.26 (3H, s, H3-16), 2.16 (1H, d, J = 11.2 Hz, H-9), 2.10 (3H, s, H3-24), 2.07 (3H, s, H3-22), 2.05 (1H, m, H2-7a), 1.37 (1H, m, H2-7b), 1.60 (1H, m, H2-2a), 1.47 (1H, m, H2-2b), 1.58 (1H, m, H2-6a), 1.36 (1H, m, H2-6b), 1.35 (1H, m, H2-1a), 0.87 (1H, m, H2-1b), 1.34 (2H, m, H2-3), 1.29 (1H, m, H-5), 1.05 (3H, s, H3-20), 0.84 (3H, s, H3-19); 13C NMR (CDCl3, 100 MHz) δC: 197.3 (C-13, s), 171.2 (C-21, s), 171.1 (C-23, s), 143.1 (C-11, d), 134.7 (C-12, d), 73.1 (C-8, s), 72.3 (C-18, t), 67.9 (C-17, t), 64.7 (C-9, d), 49.4 (C-5, d), 40.3 (C-1, t), 37.4 (C-10, s), 37.1 (C-7, t), 36.5 (C-4, s), 35.4 (C-3, t), 28.1 (C-16, q), 21.0 (C-24, q), 20.9 (C-22, q), 19.6 (C-6, t), 17.4 (C-2, t), 17.3 (C-19, q), 16.3 (C-20, q); HRESIMS m/z 412.2690 [M + NH4]+ (calcd for C22H38O6N+, 412.2694).
Crotonlaevin G (7): colorless crystal (mixed solvents: petroleum ether/MeOH/CH3CN); mp 202–203 °C; [α]26D +4.69 (c 0.64, in CHCl3); IR (KBr) νmax 3328, 2942, 2876, and 1735 cm−1; 1H NMR and 13C NMR data (CD3OD, see Tables 1 and 2); HRESIMS m/z 381.2638 [M + H]+ (calcd for C22H37O5+, 381.2636).
Crotonlaevin H (8): colorless crystal (mixed solvents: petroleum ether/CH2Cl2/EtOAc); mp 142–143 °C; [α]26D +9.09 (c 0.11, in CHCl3); IR (KBr) νmax 3399, 2932, 2873, 1733, and 1716 cm−1; 1H NMR (CDCl3, 400 MHz) δH: 5.91 (1H, dd, J = 17.6, 17.6 Hz, H-14), 5.25 (1H, d, J = 17.6, Hz, H2-15a), 5.13 (1H, d, J = 10.8 Hz, H2-15b), 4.32 (2H, d, J = 4.8, Hz, H2-17), 3.65 (1H, dd, J = 9.2, 8.0 Hz, H-12), 3.43 (1H, d, J = 10.8 Hz, H2-18a), 3.10 (1H, d, J = 10.8 Hz, H2-18b), 2.12 (3H, s, H3-22), 2.14 (1H, m, H2-7a), 1.28 (1H, m, H2-7b), 1.91 (1H, dd, J = 10.8, 5.2 Hz, H2-11a), 1.54 (1H, m, H2-11b), 1.67 (1H, m, H2-2a), 1.57 (1H, m, H2-2b), 1.64 (1H, m, H2-1a), 0.89 (1H, m, H2-1b), 1.61 (1H, m, H2-6a), 1.29 (1H, m, H2-6b), 1.57 (1H, m, H-9), 1.44 (1H, td, J = 13.2, 4.8 Hz, H2-3a), 1.25 (1H, m, H2-3b), 1.32 (1H, m, H-5), 1.30 (3H, s, H3-16), 0.87 (3H, s, H3-20), 0.74 (3H, s, H3-19); 13C NMR data (CDCl3, see Table 2); HRESIMS m/z 381.2638 [M + H]+ (calcd for C22H37O5+, 381.2636).
Crotonlaevin I (9): colorless oil; [α]26D +15.71 (c 0.13, in CHCl3); IR (KBr) νmax 3414, 2929, 2873, and 1709 cm−1; 1H NMR (CDCl3, 400 MHz) δH: 5.88 (1H, dd, J = 17.2, 17.2 Hz, H-14), 5.30 (1H, d, J = 17.2, Hz, H2-15a), 5.17 (1H, d, J = 10.8 Hz, H2-15b), 3.95 (1H, dd, J = 8.4, 7.6 Hz, H-12), 3.49 (2H, s, Hz, H2-17), 3.42 (1H, d, J = 10.8 Hz, H2-18a), 3.11 (1H, d, J = 10.8 Hz, H2-18b), 2.31 (1H, m, H2-7a), 1.24 (1H, m, H2-7b), 1.72 (1H, m, H2-6a), 1.30 (1H, m, H2-6b), 1.69 (1H, m, H2-11a), 1.66 (1H, m, H2-11b), 1.67 (1H, m, H-9), 1.66 (1H, m, H2-2a), 1.51 (1H, m, H2-2b), 1.47 (1H, m, H2-3a), 1.27 (1H, m, H2-3b), 1.42 (1H, m, H2-1a), 1.05 (1H, td, J = 11.2, 3.6 Hz, H2-1b), 1.31 (1H, m, H-5), 1.29 (3H, s, H3-16), 0.76 (6H, s, H3-19 and H3-20); 13C NMR (CDCl3, 100 MHz) δC: 140.6 (C-14, d), 114.1 (C-15, t), 83.9 (C-8, s), 82.1 (C-12, d), 74.5 (C-13, s), 71.9 (C-18, t), 60.9 (C-17, t), 60.0 (C-9, d), 50.3 (C-5, d), 38.9 (C-1, t), 37.4 (C-10, s), 36.3 (C-4, s), 35.5 (C-3, t), 33.7 (C-7, t), 24.7 (C-11, t), 24.7 (C-16, q), 19.8 (C-6, t), 17.6 (C-2, t), 17.0 (C-19, q), 15.1 (C-20, q); HRESIMS m/z 356.2793 [M + NH4]+ (calcd for C20H38NO4+, 356.2795).
15-Hydroxylabda-7,13(E)-diene-17,12-olide (10): white amorphous powder; [α]26D −2.08 (c 0.48, in CHCl3); UV λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 229 (6.87); CD (MeOH) λmax nm (Δε): 214 (−0.0095), 234 (+0.21), 268 (−0.0084); 1H NMR (CDCl3, 400 MHz) δH: 7.27 (1H, d, J = 1.6 Hz, H-7), 5.68 (1H, d, J = 6.0 Hz, H-14), 4.58 (1H, d, J = 11.6 Hz, H-12), 4.17 (2H, t, J = 3.0 Hz, H2-15), 2.33 (1H, m, H2-6a), 2.05 (1H, m, H2-6b), 2.23 (1H, d, J = 12.8 Hz, H-9), 1.90 (1H, d, J = 13.2 Hz, H2-11a), 1.45 (1H, m, H2-11b), 1.70 (1H, m, H2-1a), 1.02 (1H, td, J = 12.4, 4.0 Hz, H2-1b), 1.66 (3H, s, H3-16), 1.46 (2H, m, H2-2), 1.42 (1H, m, H2-3a), 1.15 (1H, td, J = 16.0, 4.0 Hz, H2-3b), 1.27 (1H, dd, J = 12.0, 4.8 Hz, H-5), 0.88 (3H, s, H3-19), 0.85 (3H, s, H3-18), 0.71 (3H, s, H3-20); 13C NMR (CDCl3, 100 MHz) δC: 165.8 (C-17, s), 143.1 (C-7, d), 135.5 (C-13, s), 127.4 (C-14, d), 125.7 (C-8, s), 83.7 (C-12, d), 58.7 (C-15, t), 48.9 (C-9, d), 48.7 (C-5, d), 41.7 (C-3, t), 38.5 (C-1, t), 34.6 (C-10, s), 32.7 (C-18, q), 32.7 (C-4, s), 27.1 (C-11, t), 25.0 (C-6, t), 21.3 (C-19, q), 18.4 (C-2, t), 13.3 (C-20, q), 12.0 (C-16, q); HRESIMS m/z 341.2083 [M + Na]+ (calcd for C20H30O3Na+, 341.2087).
ε): 229 (6.87); CD (MeOH) λmax nm (Δε): 214 (−0.0095), 234 (+0.21), 268 (−0.0084); 1H NMR (CDCl3, 400 MHz) δH: 7.27 (1H, d, J = 1.6 Hz, H-7), 5.68 (1H, d, J = 6.0 Hz, H-14), 4.58 (1H, d, J = 11.6 Hz, H-12), 4.17 (2H, t, J = 3.0 Hz, H2-15), 2.33 (1H, m, H2-6a), 2.05 (1H, m, H2-6b), 2.23 (1H, d, J = 12.8 Hz, H-9), 1.90 (1H, d, J = 13.2 Hz, H2-11a), 1.45 (1H, m, H2-11b), 1.70 (1H, m, H2-1a), 1.02 (1H, td, J = 12.4, 4.0 Hz, H2-1b), 1.66 (3H, s, H3-16), 1.46 (2H, m, H2-2), 1.42 (1H, m, H2-3a), 1.15 (1H, td, J = 16.0, 4.0 Hz, H2-3b), 1.27 (1H, dd, J = 12.0, 4.8 Hz, H-5), 0.88 (3H, s, H3-19), 0.85 (3H, s, H3-18), 0.71 (3H, s, H3-20); 13C NMR (CDCl3, 100 MHz) δC: 165.8 (C-17, s), 143.1 (C-7, d), 135.5 (C-13, s), 127.4 (C-14, d), 125.7 (C-8, s), 83.7 (C-12, d), 58.7 (C-15, t), 48.9 (C-9, d), 48.7 (C-5, d), 41.7 (C-3, t), 38.5 (C-1, t), 34.6 (C-10, s), 32.7 (C-18, q), 32.7 (C-4, s), 27.1 (C-11, t), 25.0 (C-6, t), 21.3 (C-19, q), 18.4 (C-2, t), 13.3 (C-20, q), 12.0 (C-16, q); HRESIMS m/z 341.2083 [M + Na]+ (calcd for C20H30O3Na+, 341.2087).
10a:1H NMR (CDCl3, 400 MHz) δH: 7.91 (2H, d, J = 8.4 Hz, H-22, 26), 7.59 (2H, d, J = 8.4 Hz, H-23, 25), 7.36 (1H, ddd, J = 4.8, 2.8, 2.8 Hz, H-7), 5.83 (1H, t, J = 6.8 Hz, H-14), 4.66 (1H, d, J = 11.8 Hz, H-12), 4.90 (2H, t, J = 6.0 Hz, H2-15), 2.39 (1H, m, H2-6a), 2.12 (1H, m, H2-6b), 2.27 (1H, m, H-9), 1.88 (1H, ddd, J = 14.2, 3.6, 1.6 Hz, H2-11a), 1.58 (1H, m, H2-11b), 1.78 (1H, m, H2-1a), 1.02 (1H, td, J = 9.2, 4.0 Hz, H2-1b), 1.82 (3H, s, H3-16), 1.53 (2H, m, H2-2), 1.48 (1H, m, H2-3a), 1.21 (1H, td, J = 12.8, 4.0 Hz, H2-3b), 1.34 (1H, dd, J = 12.0, 5.2 Hz, H-5), 0.93 (3H, s, H3-19), 0.91 (3H, s, H3-18), 0.77 (3H, s, H3-20).
Crotonlaevin J (11): white amorphous powder; [α]23D −24.00 (c 0.25, in CHCl3); IR (KBr) νmax 3417, 2926, 2867, 1711, and 1639 cm−1; UV λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 229 (7.03); CD (MeOH) λmax nm (Δε): 215 (−0.079), 235 (+0.078), 268 (−0.025); 1H NMR (CDCl3, 400 MHz) δH: 7.35 (1H, d, J = 2.0 Hz, H-7), 5.61 (1H, t, J = 6.8 Hz, H-14), 5.16 (1H, dd, J = 11.2, 2.0 Hz, H-12), 4.22 (1H, dd, J = 12.8, 7.6 Hz, H2-15a), 4.14 (1H, dd, J = 12.8, 7.6 Hz, H2-15b), 2.39 (1H, m, H2-6a), 2.10 (1H, m, H2-6b), 2.30 (1H, m, H-9), 1.80 (3H, s, H3-16), 1.75 (1H, m, H2-1a), 1.07 (1H, td, J = 12.8, 4.0 Hz, H2-1b), 1.71 (1H, m, H2-11a), 1.65 (1H, m, H2-11b), 1.59 (1H, m, H2-2a), 1.52 (1H, m, H2-2b), 1.48 (1H, m, H2-3a), 1.20 (1H, td, J = 12.8, 4.0 Hz, H2-3b), 1.32 (1H, dd, J = 12.0, 4.8 Hz, H-5), 0.93 (3H, s, H3-19), 0.90 (3H, s, H3-18), 0.77 (3H, s, H3-20); 13C NMR (CDCl3, see Table 2); HRESIMS m/z 319.2267 [M + H]+ (calcd for C20H31O3+, 319.2268).
ε): 229 (7.03); CD (MeOH) λmax nm (Δε): 215 (−0.079), 235 (+0.078), 268 (−0.025); 1H NMR (CDCl3, 400 MHz) δH: 7.35 (1H, d, J = 2.0 Hz, H-7), 5.61 (1H, t, J = 6.8 Hz, H-14), 5.16 (1H, dd, J = 11.2, 2.0 Hz, H-12), 4.22 (1H, dd, J = 12.8, 7.6 Hz, H2-15a), 4.14 (1H, dd, J = 12.8, 7.6 Hz, H2-15b), 2.39 (1H, m, H2-6a), 2.10 (1H, m, H2-6b), 2.30 (1H, m, H-9), 1.80 (3H, s, H3-16), 1.75 (1H, m, H2-1a), 1.07 (1H, td, J = 12.8, 4.0 Hz, H2-1b), 1.71 (1H, m, H2-11a), 1.65 (1H, m, H2-11b), 1.59 (1H, m, H2-2a), 1.52 (1H, m, H2-2b), 1.48 (1H, m, H2-3a), 1.20 (1H, td, J = 12.8, 4.0 Hz, H2-3b), 1.32 (1H, dd, J = 12.0, 4.8 Hz, H-5), 0.93 (3H, s, H3-19), 0.90 (3H, s, H3-18), 0.77 (3H, s, H3-20); 13C NMR (CDCl3, see Table 2); HRESIMS m/z 319.2267 [M + H]+ (calcd for C20H31O3+, 319.2268).
Crotonlaevin K (12): white amorphous powder; [α]23D +6.25 (c 0.32, in CHCl3); IR (KBr) νmax 2923, 2857, 1711, 1674, and 1638 cm−1; UV λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 234 (7.32); CD (MeOH) λmax nm (Δε): 213 (+0.014), 239 (+0.67), 270 (−0.048); 1H NMR and 13C NMR data (CDCl3, see Tables 1 and 2); HRESIMS m/z 317.2112 [M + H]+ (calcd for C20H29O3+, 317.2111).
ε): 234 (7.32); CD (MeOH) λmax nm (Δε): 213 (+0.014), 239 (+0.67), 270 (−0.048); 1H NMR and 13C NMR data (CDCl3, see Tables 1 and 2); HRESIMS m/z 317.2112 [M + H]+ (calcd for C20H29O3+, 317.2111).
Crotonlaevin L (13): white amorphous powder; [α]23D +80.00 (c 0.20, in CHCl3); IR (KBr) νmax 3385, 2927, 2869, 1705, and 1647 cm−1; UV λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 226 (6.93); CD (MeOH) λmax nm (Δε): 214 (+0.19), 239 (+0.5), 286 (−0.015); 1H NMR (acetone-D6, 400 MHz) δH: 6.94 (1H, t, J = 2.8 Hz, H-7), 5.69 (1H, t, J = 6.0 Hz, H-14), 4.72 (1H, d, J = 11.2 Hz, H-12), 4.35 (1H, dt, J = 10.4, 2.8 Hz, H-6), 4.13 (2H, t, J = 5.6 Hz, H2-15), 2.44 (1H, dq, J = 13.2, 3.2 Hz, H-9), 1.87 (1H, ddd, J = 13.2, 3.2, 2.0 Hz, H2-11a), 1.49 (1H, m, H2-11b), 1.72 (1H, m, H2-1a), 1.17 (1H, m, H2-1b), 1.59 (1H, dt, J = 13.6, 3.2 Hz, H2-2a), 1.47 (1H, m, H2-2b), 1.67 (3H, s, H3-16), 1.43 (1H, m, H2-3a), 1.26 (1H, td, J = 13.6, 3.2 Hz, H2-3b), 1.39 (1H, m, H-5), 1.17 (3H, s, H3-18), 1.06 (3H, s, H3-19), 0.84 (3H, s, H3-20); 13C NMR (acetone-D6, see Table 2); HRESIMS m/z 357.2025 [M + Na]+ (calcd for C20H30O4Na+, 357.2034).
ε): 226 (6.93); CD (MeOH) λmax nm (Δε): 214 (+0.19), 239 (+0.5), 286 (−0.015); 1H NMR (acetone-D6, 400 MHz) δH: 6.94 (1H, t, J = 2.8 Hz, H-7), 5.69 (1H, t, J = 6.0 Hz, H-14), 4.72 (1H, d, J = 11.2 Hz, H-12), 4.35 (1H, dt, J = 10.4, 2.8 Hz, H-6), 4.13 (2H, t, J = 5.6 Hz, H2-15), 2.44 (1H, dq, J = 13.2, 3.2 Hz, H-9), 1.87 (1H, ddd, J = 13.2, 3.2, 2.0 Hz, H2-11a), 1.49 (1H, m, H2-11b), 1.72 (1H, m, H2-1a), 1.17 (1H, m, H2-1b), 1.59 (1H, dt, J = 13.6, 3.2 Hz, H2-2a), 1.47 (1H, m, H2-2b), 1.67 (3H, s, H3-16), 1.43 (1H, m, H2-3a), 1.26 (1H, td, J = 13.6, 3.2 Hz, H2-3b), 1.39 (1H, m, H-5), 1.17 (3H, s, H3-18), 1.06 (3H, s, H3-19), 0.84 (3H, s, H3-20); 13C NMR (acetone-D6, see Table 2); HRESIMS m/z 357.2025 [M + Na]+ (calcd for C20H30O4Na+, 357.2034).
Crotonlaevin M (14): white amorphous powder; [α]26D +10.00 (c 0.10, in CHCl3); IR (KBr) νmax 3381, 3296, 2944, 2920, 2849, 1683, and 1625 cm−1; UV λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 221 (6.88); CD (MeOH) λmax nm (Δε): 217 (−0.79), 256 (−0.28); 1H NMR (C5D5N, 400 MHz) δH: 6.11 (1H, t, J = 6.0 Hz, H-14), 5.25 (1H, d, J = 4.0 Hz, H-7), 4.74 (1H, dd, J = 12.0, 3.6 Hz, H-12), 4.53 (2H, d, J = 6.0 Hz, H2-15), 2.48 (1H, ddd, J = 17.2, 12.0, 1.2 Hz, H2-11a), 2.36 (1H, dd, J = 17.2, 3.6 Hz, H2-11b), 2.11 (1H, d, J = 13.6 Hz, H2-6a), 1.68 (1H, dd, J = 13.6, 4.4 Hz, H2-6b), 1.96 (1H, dd, J = 12.8, 1.2 Hz, H-5), 1.78 (3H, s, H3-16), 1.68 (1H, m, H2-1a), 1.07 (1H, td, J = 12.8, 3.6 Hz, H2-1b), 1.58 (1H, m, H2-2a), 1.47 (1H, m, H2-2b), 1.40 (1H, d, J = 13.6 Hz, H2-3a), 1.17 (1H, td, J = 12.8, 3.6 Hz, H2-3b), 1.03 (3H, s, H3-18), 0.92 (3H, s, H3-20), 0.85 (3H, s, H3-19); 13C NMR data (C5D5N, see Table 2); HRESIMS m/z 357.2034 [M + Na]+ (calcd for C20H30O4Na+, 357.2036).
ε): 221 (6.88); CD (MeOH) λmax nm (Δε): 217 (−0.79), 256 (−0.28); 1H NMR (C5D5N, 400 MHz) δH: 6.11 (1H, t, J = 6.0 Hz, H-14), 5.25 (1H, d, J = 4.0 Hz, H-7), 4.74 (1H, dd, J = 12.0, 3.6 Hz, H-12), 4.53 (2H, d, J = 6.0 Hz, H2-15), 2.48 (1H, ddd, J = 17.2, 12.0, 1.2 Hz, H2-11a), 2.36 (1H, dd, J = 17.2, 3.6 Hz, H2-11b), 2.11 (1H, d, J = 13.6 Hz, H2-6a), 1.68 (1H, dd, J = 13.6, 4.4 Hz, H2-6b), 1.96 (1H, dd, J = 12.8, 1.2 Hz, H-5), 1.78 (3H, s, H3-16), 1.68 (1H, m, H2-1a), 1.07 (1H, td, J = 12.8, 3.6 Hz, H2-1b), 1.58 (1H, m, H2-2a), 1.47 (1H, m, H2-2b), 1.40 (1H, d, J = 13.6 Hz, H2-3a), 1.17 (1H, td, J = 12.8, 3.6 Hz, H2-3b), 1.03 (3H, s, H3-18), 0.92 (3H, s, H3-20), 0.85 (3H, s, H3-19); 13C NMR data (C5D5N, see Table 2); HRESIMS m/z 357.2034 [M + Na]+ (calcd for C20H30O4Na+, 357.2036).
Crotonlaevin N (15): colorless crystal (mixed solvents: petroleum ether/EtOAc/acetone); mp 125–127 °C; [α]23D −18.75 (c 0.32, in CHCl3); IR (KBr) νmax 3427, 2926, 2869, 1707, and 1641 cm−1; UV λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 223 (6.85); CD (MeOH) λmax nm (Δε): 219 (−0.44), 254 (+0.057); 1H NMR and 13C NMR data (CDCl3, see Tables 1 and 2); HRESIMS m/z 319.2257 [M + H]+ (calcd for C20H31O3+, 319.2268).
ε): 223 (6.85); CD (MeOH) λmax nm (Δε): 219 (−0.44), 254 (+0.057); 1H NMR and 13C NMR data (CDCl3, see Tables 1 and 2); HRESIMS m/z 319.2257 [M + H]+ (calcd for C20H31O3+, 319.2268).
Crotonlaevin O (16): colorless crystal (mixed solvents: petroleum ether/EtOAc); mp 167–168 °C; [α]20D +30.00 (c 0.3, in CHCl3); IR (KBr) νmax 3428, 2927, 2850, 1703, and 1640 cm−1; UV λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 228 (6.66); CD (MeOH) λmax nm (Δε): 230 (+0.24), 264 (−0.019); 1H NMR (CDCl3, 400 MHz) δH: 7.33 (1H, dd, J = 2.4, 4.4 Hz, H-7), 5.96 (1H, dd, J = 17.2, 17.2 Hz, H-14), 5.40 (1H, d, J = 17.2 Hz, H2-15a), 5.22 (1H, d, J = 10.8 Hz, H2-15b), 4.12 (1H, dd, J = 10.8, 1.6 Hz, H-12), 2.38 (1H, m, H2-6a), 2.11 (1H, m, H2-6b), 2.16 (1H, m, H-9), 1.93 (1H, m, H2-11a), 1.34 (1H, m, H2-11b), 1.79 (1H, m, H2-1a), 1.06 (1H, td, J = 12.8, 4.0 Hz, H2-1b), 1.58 (1H, m, H2-2a), 1.53 (1H, m, H2-2b), 1.48 (1H, m, H2-3a), 1.20 (1H, td, J = 12.8, 4.0 Hz, H2-3b), 1.35 (3H, s, H3-16), 1.32 (1H, m, H-5), 0.92 (3H, s, H3-19), 0.90 (3H, s, H3-18), 0.75 (3H, s, H3-20); 13C NMR data (CDCl3, see Table 2); HRESIMS m/z 319.2261 [M + H]+ (calcd for C20H31O3+, 319.2268).
ε): 228 (6.66); CD (MeOH) λmax nm (Δε): 230 (+0.24), 264 (−0.019); 1H NMR (CDCl3, 400 MHz) δH: 7.33 (1H, dd, J = 2.4, 4.4 Hz, H-7), 5.96 (1H, dd, J = 17.2, 17.2 Hz, H-14), 5.40 (1H, d, J = 17.2 Hz, H2-15a), 5.22 (1H, d, J = 10.8 Hz, H2-15b), 4.12 (1H, dd, J = 10.8, 1.6 Hz, H-12), 2.38 (1H, m, H2-6a), 2.11 (1H, m, H2-6b), 2.16 (1H, m, H-9), 1.93 (1H, m, H2-11a), 1.34 (1H, m, H2-11b), 1.79 (1H, m, H2-1a), 1.06 (1H, td, J = 12.8, 4.0 Hz, H2-1b), 1.58 (1H, m, H2-2a), 1.53 (1H, m, H2-2b), 1.48 (1H, m, H2-3a), 1.20 (1H, td, J = 12.8, 4.0 Hz, H2-3b), 1.35 (3H, s, H3-16), 1.32 (1H, m, H-5), 0.92 (3H, s, H3-19), 0.90 (3H, s, H3-18), 0.75 (3H, s, H3-20); 13C NMR data (CDCl3, see Table 2); HRESIMS m/z 319.2261 [M + H]+ (calcd for C20H31O3+, 319.2268).
Crotonlaevin P (17): colorless crystal (mixed solvents: petroleum ether/EtOAc/MeOH); mp 122–124 °C; [α]23D +32.00 (c 0.25, in CHCl3); IR (KBr) νmax 3432, 2927, 2851, 1706, and 1640 cm−1; UV λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 230 (6.97); CD (MeOH) λmax nm (Δε): 208 (+0.032), 232 (+0.41), 268 (−0.024); 1H NMR (CDCl3, 400 MHz) δH: 7.33 (1H, m, H-7), 5.89 (1H, dd, J = 17.3, 17.3 Hz, H-14), 5.38 (1H, dd, J = 17.3, 0.8 Hz, H2-15a), 5.21 (1H, d, J = 10.8, 0.8 Hz, H2-15b), 4.10 (1H, dd, J = 10.8, 1.6 Hz, H-12), 2.37 (1H, m, H2-6a), 2.09 (1H, m, H2-6b), 2.16 (1H, m, H-9), 1.87 (1H, m, H2-11a), 1.41 (1H, m, H2-11b), 1.77 (1H, m, H2-1a), 1.04 (1H, td, J = 12.0, 4.0 Hz, H2-1b), 1.57 (1H, m, H2-2a), 1.51 (1H, m, H2-2b), 1.47 (1H, m, H2-3a), 1.18 (1H, td, J = 12.0, 4.0 Hz, H2-3b), 1.38 (3H, s, H3-16), 1.29 (1H, dd, J = 12.0, 4.0 Hz, H-5), 0.91 (3H, s, H3-19), 0.89 (3H, s, H3-18), 0.74 (3H, s, H3-20); 13C NMR data (CDCl3, see Table 2); HRESIMS m/z 341.2085 [M + Na]+ (calcd for C20H30NaO3+, 341.2087).
ε): 230 (6.97); CD (MeOH) λmax nm (Δε): 208 (+0.032), 232 (+0.41), 268 (−0.024); 1H NMR (CDCl3, 400 MHz) δH: 7.33 (1H, m, H-7), 5.89 (1H, dd, J = 17.3, 17.3 Hz, H-14), 5.38 (1H, dd, J = 17.3, 0.8 Hz, H2-15a), 5.21 (1H, d, J = 10.8, 0.8 Hz, H2-15b), 4.10 (1H, dd, J = 10.8, 1.6 Hz, H-12), 2.37 (1H, m, H2-6a), 2.09 (1H, m, H2-6b), 2.16 (1H, m, H-9), 1.87 (1H, m, H2-11a), 1.41 (1H, m, H2-11b), 1.77 (1H, m, H2-1a), 1.04 (1H, td, J = 12.0, 4.0 Hz, H2-1b), 1.57 (1H, m, H2-2a), 1.51 (1H, m, H2-2b), 1.47 (1H, m, H2-3a), 1.18 (1H, td, J = 12.0, 4.0 Hz, H2-3b), 1.38 (3H, s, H3-16), 1.29 (1H, dd, J = 12.0, 4.0 Hz, H-5), 0.91 (3H, s, H3-19), 0.89 (3H, s, H3-18), 0.74 (3H, s, H3-20); 13C NMR data (CDCl3, see Table 2); HRESIMS m/z 341.2085 [M + Na]+ (calcd for C20H30NaO3+, 341.2087).
X-ray single-crystal diffraction analysis of 7, 8, 10a, 14, 15, 16, and 17
All data were collected using a Bruker Smart Apex CCD diffractometer using graphite-monochromated Mo Kα and Cu Kα radiation. The structure was solved by the direct method SHELXS-97 (Sheldrick, G.M., University of Gottingen, Gottingen, Germany, 1997) and refined by a full-matrix least-square method on F2 by means of SHELXL-97 (Sheldrick, G.M., University of Gottingen, Gottingen, Germany, 1997). The hydrogen atoms were not included in the refinement, and all of the non-hydrogen atoms were refined anisotropically. In the final step of structural refinement, the positional parameters of the hydrogen atoms were calculated under a fixed C–H bond length of 1.00 Å with sp3 configuration of the bonding carbon atoms.†Crystal data of 7: Cu Kα radiation (294 K):C22H36O5; F.W. 380.51; monoclinic space group I2; unit cell dimensions a = 20.3298(3) Å, b = 9.17075(12) Å, c = 24.6190(4) Å, V = 4233.70(12) Å3; α = 90°, β = 112.7227(19)°, γ = 90°, Z = 8, ρcalc = 1.194 Mg m−3, crystal dimensions 0.2 × 0.08 × 0.06 mm, μ = 0.665 mm−1, F(000) = 1664. A total of 20139 reflections were measured, of which 7487 were unique (Rint = 0.0274) and used in all calculations. The final refinement gave R1 = 0.0411 and wR2 = 0.1160 [I > 2σ(I)].
Crystal data of 8: Mo Kα radiation (296 K):C22H36O5; F.W. 380.51; monoclinic space group C2; unit cell dimensions a = 26.328(15) Å, b = 11.042(7) Å, c = 19.217(19) Å, V = 4336(6) Å3; α = 90°, β = 129.100(4)°, γ = 90°, Z = 8, ρcalc = 1.166 Mg m−3, crystal dimensions 0.23 × 0.22 × 0.19 mm, μ = 0.081 mm−1, F(000) = 1664. A total of 14628 reflections were measured, of which 7876 were unique (Rint = 0.0507) and used in all calculations. The final refinement gave R1 = 0.0785 and wR2 = 0.1415 [I > 2σ(I)].
Crystal data of 10a: Mo Kα radiation (296 K):C22H33BrO4; F.W. 501.44; monoclinic space group P2(1); unit cell dimensions a = 11.718(16) Å, b = 8.241(11) Å, c = 13.602(18) Å, V = 1251(3) Å3; α = 90°, β = 107.699(13)°, γ = 90°, Z = 2, ρcalc = 1.331 Mg m−3, crystal dimensions 0.23 × 0.21 × 0.19 mm, μ = 1.672 mm−1, F(000) = 524. A total of 6336 reflections were measured, of which 4296 were unique (Rint = 0.0387) and used in all calculations. The final refinement gave R1 = 0.0498 and wR2 = 0.0982 [I > 2σ(I)].
Crystal data of 14: Cu Kα radiation (290 K):C20H30O4; F.W. 334.44; monoclinic space group C2; unit cell dimensions a = 9.1613(4) Å, b = 11.8102(7) Å, c = 16.9960(9) Å, V = 1829.40(17) Å3; α = 90°, β = 95.829(5)°, γ = 90°, Z = 4, ρcalc = 1.214 Mg m−3, crystal dimensions 0.29 × 0.25 × 0.24 mm, μ = 0.663 mm−1, F(000) = 728. A total of 17362 reflections measured, of which 3503 were unique (Rint = 0.0334) and used in all calculations. The final refinement gave R1 = 0.0341 and wR2 = 0.0917 [I > 2σ(I)].
Crystal data of 15: Cu Kα radiation (291 K):C20H30O3; F.W. 318.44; orthorhombic space group P2(1)2(1)2(1); unit cell dimensions a = 7.1319(6) Å, b = 14.3359(11) Å, c = 17.4859(15) Å, V = 1787.8(3) Å3; α = 90°, β = 90°, γ = 90°, Z = 4, ρcalc = 1.183 Mg m−3, crystal dimensions 0.25 × 0.14 × 0.1 mm, μ = 0.611 mm−1, F(000) = 696. A total of 4991 reflections were measured, of which 2976 were unique (Rint = 0.0183) and used in all calculations. The final refinement gave R1 = 0.0374 and wR2 = 0.0977 [I > 2σ(I)].
Crystal data of 16: Mo Kα radiation (296 K):C20H30O3; F.W. 318.44; orthorhombic space group P2(1)2(1)2(1); unit cell dimensions a = 15.646(17) Å, b = 10.703(12) Å, c = 10.876(12) Å, V = 1821(3) Å3; α = 90°, β = 90°, γ = 90°, Z = 4, ρcalc = 1.161 Mg m−3, crystal dimensions 0.23 × 0.21 × 0.19 mm, μ = 0.076 mm−1, F(000) = 696. A total of 12994 reflections were measured, of which 3376 were unique (Rint = 0.0499) and used in all calculations. The final refinement gave R1 = 0.0391 and wR2 = 0.0839 [I > 2σ(I)].
Crystal data of 17: Mo Kα radiation (296 K):C20H30O3; F.W. 318.44; orthorhombic space group P2(1)2(1)2(1); unit cell dimensions a = 6.519(2) Å, b = 17.905(6) Å, c = 31.128(10) Å, V = 3633.2(19) Å3; α = 90°, β = 90°, γ = 90°, Z = 8, ρcalc = 1.164 Mg m−3, crystal dimensions 0.28 × 0.26 × 0.21 mm, μ = 0.076 mm−1, F(000) = 1392. A total of 26369 reflections were measured, of which 6748 were unique (Rint = 0.1056) and used in all calculations. The final refinement gave R1 = 0.0809 and wR2 = 0.1654 [I > 2σ(I)].
Acknowledgements
Financial support from the National Natural Science Foundation of China (no. 21202075), the National Science Foundation for Fostering Talents in Basic Research of the National Natural Science Foundation of China (J1103307), and the Fundamental Research Funds for the Central Universities (lzujbky-2014-64) is gratefully acknowledged.References
- P. M. Giang, P. T. Son, Y. Hamada and H. Otsuka, Chem. Pharm. Bull., 2005, 53, 296–300 CrossRef CAS.
- S. Roengsumran, K. Musikul, A. Petsom, T. Vilaivan, P. Sangvanich, S. Pornpakakul, S. Puthong, C. Chaichantipyuth, N. Jaiboon and N. Chaichit, Planta Med., 2002, 68, 274–277 CrossRef CAS PubMed.
- J. Thongtan, P. Kittakoop, N. Ruangrungsi, J. Saenboonrueng and Y. Thebtaranonth, J. Nat. Prod., 2003, 66, 868–870 CrossRef CAS PubMed.
- S. Athikomkulchai, H. Prawat, N. Thasana, N. Ruangrungsi and S. Ruchirawat, Chem. Pharm. Bull., 2006, 54, 262–264 CrossRef CAS.
- P. C. Kuo, Y. C. Shen, M. L. Yang, S. H. Wang, T. D. Thang, N. X. Dung, P. C. Chiang, K. H. Lee, E. J. Lee and T. S. Wu, J. Nat. Prod., 2007, 70, 1906–1909 CrossRef CAS PubMed.
- X. A. Wu and Y. M. Zhao, Nat. Prod. Res. Dev., 2004, 16, 467–472 CAS.
- M. K. Langat, N. R. Crouch, P. J. Smith and D. A. Mulholland, J. Nat. Prod., 2011, 74, 2349–2355 CrossRef CAS PubMed.
- G. A. Zou, G. Ding, Z. H. Su, J. S. Yang, H. W. Zhang, C. Z. Peng, H. A. Aisa and Z. M. Zou, J. Nat. Prod., 2010, 73, 792–795 CrossRef CAS PubMed.
- C. Vigor, N. Fabre, I. Fouraste and C. Moulis, J. Nat. Prod., 2002, 65, 1180–1182 CrossRef CAS PubMed.
- O. L. Rakotonandrasana, F. H. Raharinjato, M. Rajaonarivelo, V. Dumontet, M. T. Martin, J. Bignon and P. Rasoanaivo, J. Nat. Prod., 2010, 73, 1730–1733 CrossRef CAS PubMed.
- G. C. Wang, H. Zhang, H. B. Liu and J. M. Yue, Org. Lett., 2013, 15, 4880–4883 CrossRef CAS PubMed.
- Jiangsu New Medical College, Dictionary of Chinese Materria Medica, Shanghai Science & Technology Publishing House, Shanghai, 1975, pp. 881–882 Search PubMed.
- C. S. Li, J. L. Zhao, L. X. Zhang and W. W. Gao, Zhongchengyao, 2011, 33, 1740–1742 CAS.
- R. Nagel, Altex-Altern Tierexp, 2002, 19, 38–48 Search PubMed.
- S. W. Pelletier, S. Lajsic, Y. Ohtsuka and Z. Djarmati, J. Org. Chem., 1975, 40, 1607–1609 CrossRef CAS.
- X. W. Yang, S. M. Li, L. Feng, Y. H. Shen, J. M. Tian, X. H. Liu, H. W. Zeng, C. Zhang and W. D. Zhang, Tetrahedron, 2008, 64, 4354–4362 CrossRef CAS PubMed.
- M. I. Ybarra, S. Popich, S. A. Borkosky, Y. Asakawa and A. Bardon, J. Nat. Prod., 2005, 68, 554–558 CrossRef CAS PubMed.
- H. D. Flack, Acta Crystallogr., Sect. A: Found. Crystallogr., 1983, 39, 876–881 CrossRef.
- H. D. Flack and G. Bernardinelli, Chirality, 2008, 20, 681–690 CrossRef CAS PubMed.
- D. Sommit, A. Petsom, T. Ishikawa and S. Roengsumran, Planta Med., 2003, 69, 167–170 CrossRef CAS PubMed.
- N. Berova; K. Nakanishi, in Circular Dichroism: Principles and Applications, ed. Berova, N., Nakanishi, K., Woody, R. W., Wiley-VCH, New York, 2nd edn, 2000, pp. 337–382 Search PubMed.
- J. L. Yang and Y. P. Shi, Phytochemistry, 2012, 76, 124–132 CrossRef CAS PubMed.
- T. Yuan, S. P. Yang, C. R. Zhang, S. Zhang and J. M. Yue, Org. Lett., 2009, 11, 617–620 CrossRef CAS PubMed.
- I. S. Marcos, R. F. Moro, S. Carballares and J. G. Urones, Tetrahedron, 2001, 57, 713–723 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: HRESIMS, IR, UV, CD, and NMR spectra of 1–17, and X-ray crystallographic in CIF data for 7, 8, 10a, and 14–17. Selected key HMBC correlations for new compounds 1–9 and 11–17. CCDC 1002242–1002248. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra04863f |
| This journal is © The Royal Society of Chemistry 2014 |