Copper nanowire synthesis by directed electrochemical nanowire assembly†
Abstract
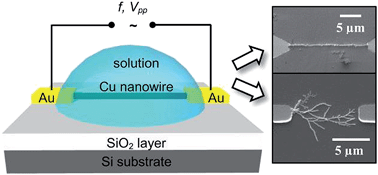
We present the growth of high aspect ratio copper nanowires with diameters of about 100 nm and a length of up to several micrometers via directed electrochemical nanowire assembly (DENA). We demonstrate that the nanowire morphology is controlled by the metal salt solution concentration and by the amplitude and frequency of the applied alternating voltage. The frequency was found to notably affect the nanowire diameter. The impact of the process parameters on the nanowire growth is discussed on the basis of the Butler–Volmer equation. The presented results for the bottom-up fabrication of copper wires are of high interest for nanosensor design, particularly for developing highly sensitive gas sensors. We suggest guidelines for the formation of copper nanowires based on experimental and theoretical considerations.

 Please wait while we load your content...
Please wait while we load your content...