Improvement of wood properties by impregnation with TiO2via ultrasonic-assisted sol–gel process
Abstract
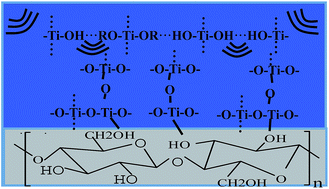
Wood with improved thermal and mechanical properties as well as dimensional stability, was successfully prepared by modification with TiO2. In this paper, we report an innovative and simple method, namely, the ultrasonic-assisted sol–gel method, to prepare TiO2-modified wood. The formation mechanism of TiO2-modified wood is also presented. After sonication under mild conditions, weight percent gains (WPGs) of wood samples in the range of 18–30% were obtained. The WPGs increased with increasing ultrasonic irradiation time of up to 120 min, after which the WPGs increased slightly then hardly changed. The functional groups and morphology of the synthesized modified wood were examined by Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM) with built-in energy-dispersive X-ray analysis. SEM investigations showed that the majority of TiO2 particles deposited in lumen by adhesion to cell walls in the form of aggregates. The thermal and mechanical performances as well as moisture absorption behavior of the generated modified wood materials were also evaluated.

 Please wait while we load your content...
Please wait while we load your content...