Aqueous dispersion of polymer coated boron nitride nanotubes and their antibacterial and cytotoxicity studies
Jeghan Shrine Maria Nithyaa and
Arumugam Pandurangan*ab
aDepartment of Chemistry, Anna University, Chennai-600025, Tamilnadu, India
bInstitute of Catalysis and Petroleum Technology, Anna University, Chennai, Tamilnadu, India. E-mail: pandurangan_a@yahoo.com; Fax: +91-44-2220060; Tel: +91-44-22358653
First published on 15th July 2014
Abstract
The present study focuses on the aqueous dispersion of BNNTs via noncovalent functionalization with four surfactants including pluronic (P123), polyethyleneimine (PEI), pluronic (F127) and ammonium oleate (A.O). The morphology of the modified BNNTs was characterized by transmission electron microscopy (TEM). Pristine BNNTs and surfactant coated BNNTs were examined to elucidate their antibacterial activity and cytotoxicity levels in different cells (Vero, Chang liver, MCF7 and A549) by an in vitro method. The quantitative antibacterial efficacy was evaluated by measuring the optical density growth of a bacterial culture. The optical density growth curves and TEM results indicate that PEI coated BNNTs exhibit strong bacterial activity against Escherichia coli and Staphylococcus aureus. The cytotoxicity levels were intensively studied by the MTT method and the results reveal the biocompatible nature of F127 coated BNNTs (F-BNNTs) in different cells. The chemotherapeutic drugs, tamoxifen and paclitaxel blended F-BNNTs were tested in MCF7 and A549 cells. DNA fragmentation and fluorescence images indicate the apoptotic pathway of cell death in cancer cells. The results of this investigation explore the cytocompatability of F-BNNTs and it can be designed as a nanovector for targeted drug delivery.
1. Introduction
The attractive properties of nanomaterials have paved the way for a wide range of applications in technology, research, and medicine. Nanomaterials including nano-capsules, nanoparticles, nanotubes, nanowires and nanofibers have multifaceted applications in biomedical research.1 Among various one dimensional nanomaterials, carbon nanotubes (CNTs) and boron nitride nanotubes (BNNTs) have unique properties that can be exploited as probes for diagnostic purposes, biological detection, imaging and drug delivery in cancer.2 Owing to their structural similarities, BNNTs have superior properties than CNTs due to their robust structure which resists high temperatures and harsh chemical modifications.3,4 CNTs have been synthesised by three main techniques namely arc discharge, laser ablation and chemical vapour deposition (CVD). In BNNT synthesis, the high yield and purity was not achieved with these three popular methods. This is the major drawback that BNNTs applications in various fields were not explored than CNTs.5Alternative synthesis methods such as substitution reactions, ball milling, plasma jet, metal-boride-catalyzed CVD, low- or high temperature chemical synthesis, plasma enhanced pulsed laser deposition (PE-PLD) and boron oxide chemical vapor deposition (BOCVD) enhances the yield of BNNTs with certain limitations.6 The recent reports on BNNTs synthesis by catalytic chemical vapor deposition (CCVD), thermal annealing, improved laser heating and improved ball milling methods yields large quantities of nanotubes.7–12
CNTs are widely used in biomedical applications and their results confirm that the pristine CNTs were toxic and functionalised CNTs are non-toxic. The functionalized CNTs improve their biocompatibility with different types of cells.13,14 On the other hand, pristine (purified) BNNTs were found to be nontoxic, but the hindrance of solubility restricts their biocompatibility. Covalent and non-covalent functionalization by the attachment of functional or polymeric groups modifies the surface of nanotubes enables BNNT to solubilise in various solvents.
Covalent linking of BNNTs with stearoyl chloride, diamine terminated polyethylene glycol, ammonia plasma treated, 3-aminopropyl-triethoxysilane (APTES), isocyanates, H2O2 oxidation treatment permits BNNTs solubilisation in different solvents.15–19 The chemical approaches involved in covalent functionalization need high pressure or high vacuum condition. These might create defects to the BN network and leads to a major alteration of its properties. In contrast, a non-covalent functionalization method involves mild process like ultrasonication, centrifugation and filtration. The main advantage of noncovalent approach is that it is not destructive for the conjugation of hexagonal BN lattice.
Non-covalent functionalisation with proteins, polynucleotides, glycodendrimers, polyethyleneimine, poly peptides, poly-L-lysine, glycol-chitosan, PEGylated phospholipid, polymeric materials (amine-terminated polyethylene glycol (PEG)), polyoxypropylene diamine (OPA), randomly oxidized polyethylene (oxi-PE) and biopolymer-glycine wrappings enables dispersion and stabilisation of BNNTs in aqueous medium for long period.20–29
It should be emphasised that the first biocompatibility findings of BNNTs with human neuroblastoma cells (SH-SY5Y) revealed BNNT does not have adverse effects on viability, metabolism and cellular replication.30 In vitro studies on BNNTs with different cells includes human embryonic kidney (HEK 293), Chinese hamster ovary cells (CHO), mouse myoblasts (C2C12), neuronal (PC12) and glioblastoma multiforme (T98G) cells explore the biocompatible nature of BNNTs.31 Further studies on osteoblasts, macrophages, malignant U87, T98 (mutant p53) glioblastoma, MCF-7 adenocarcinoma mammary gland cells, and normal MRC (diploid) fibroblasts cells also confirmed the non toxic nature of BNNTs.32 The excellent proliferation, metabolic activity at lower concentration suggests that BNNTs can be used as a nanovector in cell therapy and drug delivery system. In addition to these reports, the in vivo studies in rabbits also evidently confirm the non-toxic characteristics of BNNTs.33,34
In this work, BNNTs synthesised by annealing boron, V2O5 and Ni2O3 mixtures in nitrogen/ammonia gas atmosphere at 1100 °C were utilized for noncovalent functionalisation. The as-synthesised BNNTs were acid purified and coated with surfactants (P123, A.O, PEI and F127) that have capacities to adsorb hydrophobic surfaces. Such kind of functionalities has the ability to suspend BNNTs individually by imparting a distribution of charges onto the BN surface that prevents their re-aggregation. To the best of our knowledge for the first time, in our study pristine BNNTs and coated BNNTs were applied in Gram negative Escherichia coli (E. coli) and Gram positive Staphylococcus aureus (S. aureus) bacteria to check their antibacterial activity. However, preliminary studies with these bacterial species were performed in order to check its cytotoxic nature with different cells. The cytotoxic nature of pristine BNNTs, BNNTs coated with P123, A.O, PEI and F127 were tested in Vero, Chang human liver cells, MCF7 and A549 cells by in vitro method. The percentage of cell viability was determined by MTT assay. In addition, DNA fragmentation and fluorescence studies were also carried out to confirm the cell death.
2. Materials and methods
2.1 Materials
Amorphous boron powder (B) (Loba Chemie. Pvt. Ltd), Vanadium pentoxide (V2O5) and Nickel oxide (Ni2O3) (Sisco Research Laboratories Pvt. Ltd) were purchased and used as such. Ammonia and nitrogen gases were used as nitrogen source and carrier gases, respectively. Pluronic P123, polyethyleneimine (PEI) and pluronic F127 were kindly supplied by Sigma Aldrich Pvt. Ltd. Oleic acid and ammonia solutions were purchased from Sisco Research Laboratories Pvt. Ltd.E. coli and S. aureus cultures were kindly provided by King's Institute of Preventive medicine, Chennai, India. Nutrient Broth (NB), from Sigma Aldrich Pvt. Ltd, was purchased and used as such. Cell lines namely Green monkey kidney Vero cells (kidney epithelial cell line of the African Green Monkey), human normal liver cells (Chang), adenocarcinoma mammary gland cells (MCF-7) and lung epithelial cells (A-549) were obtained from Vepery medical college and hospital, National center for cell science (NCCS, Pune) and King's Institute of Preventive medicine, Chennai, India. Commercially available lung cancer drug paclitaxel and breast cancer drug tamoxifen were purchased from Adley formulations (pharmaceutical company in India).
2.2 Synthesis of BNNTs
BNNTs were synthesised by thermal heating chemical vapor deposition (CVD) method as reported in our previous work.35 In brief, mixture of amorphorous boron powder, Ni2O3 and V2O5 were loaded in alumina boat and kept in the center of the horizontal furnace made up of alumina tube. The furnace were programmed from ambient to 1100 °C kept for 1 h in N2/NH3 gas atmosphere at rap rate 5 °C min−1 and cooled under N2 gas flow. The as-synthesised product were purified using HCl and HNO3 to remove amorphous boron and metal oxide impurities.362.3 BNNTs dispersion and functionalisation
The dispersion and functionalisation of BNNTs was adopted as reported by Ciofani et al. 2008.24 BNNTs of 5 mg were suspended in 0.1% (10 mL) of P123 and sonicated for 6 h for even dispersion of BNNTs followed by stirring at 70 °C for 6 h. The suspended solutions were centrifuged at 5000 rpm to remove the non dispersed residual impurities. The excess of polymeric solutions were also removed by centrifuging for more than five times at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and vacuum dried at 50 °C. The same procedures were followed to coat BNNTs with PEI and F127. The ammonium oleate and its suspensions were prepared by the typical procedure mixing 1 mL of oleic acid and 1 mL of concentrated ammonia solutions.37 The thick surfactant was further diluted with distilled water. BNNTs dispersion were prepared by sonicating 10 mL of ammonium oleate with 5 mg of BNNTs for 12 h and the non dispersed residual impurities and heavier BNNTs particles were removed by centrifugation process.
000 rpm and vacuum dried at 50 °C. The same procedures were followed to coat BNNTs with PEI and F127. The ammonium oleate and its suspensions were prepared by the typical procedure mixing 1 mL of oleic acid and 1 mL of concentrated ammonia solutions.37 The thick surfactant was further diluted with distilled water. BNNTs dispersion were prepared by sonicating 10 mL of ammonium oleate with 5 mg of BNNTs for 12 h and the non dispersed residual impurities and heavier BNNTs particles were removed by centrifugation process.
2.4 Preparation of bacterial cells
The scheme adopted to evaluate the antibacterial activity and the details were reported elsewhere (Bai et al. 2011).38 In brief, the pure cultures of E. coli and S. aureus were inoculated into the nutrient broth (NB) separately and kept for incubation in a rotary shaker at 200 rpm for 16 h at 37 °C. The cells were washed and suspended in saline solution (0.9% NaCl) to remove the nutrients from the growth media. About 150 μL of E. coli and S. aureus samples were treated with 50 μL of polymer coated BNNTs with desired concentrations (0.1, 0.2, 0.5 and 1.0 mg mL−1). Control samples of both the cultures each 150 μL were added with 50 μL distilled water separately and maintained for desired period of time. These samples were kept in a shaker at 200 rpm at room temperature. The pristine BNNTs suspension and polymeric solutions of 0.5 mg mL−1 were also examined following the same procedure. After treatment the samples were transferred to 4 mL of NB and the samples were kept in an incubator at 37 °C. The bacterial cell growth was monitored by measuring optical density (OD) values at 600 nm for every 2 h on a spectrophotometer. The growth graphs were obtained by plotting OD values verses regular intervals time.2.5 Cell culture
The cell culture and the protocol for the maintenance of cells were followed as reported by (Ciofani et al. 2008).24 Vero cells, Chang cells, MCF-7 and A549 were grown in culture flasks and maintained in Minimal Essential Media (MEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U mL−1), and streptomycin (100 μg mL−1) at 37 °C in 5% CO2 incubator. The cells were subcultured by trypsination and maintained by frequent change of media for every 2–3 days.Cells from the sub cultured flasks were transferred to 24 well plates (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per well) and allowed to grow well for two days at 37 °C to reach a confluence of 90% in 5% CO2 incubator. The stock solutions of pristine BNNT, P123, A.O, PEI, F127, P123-BNNT, A.O-BNNT, PEI-BNNT and F127-BNNT were serial diluted in MEM medium (1000, 500, 250, 125, 62.5, 31.25, 15.62, 7.8 μg mL−1). These samples were labelled as BNNT, P, A.O, PEI, F, P-BNNT, A.O-BNNT, PEI-BNNT and F-BNNT, respectively.
000 per well) and allowed to grow well for two days at 37 °C to reach a confluence of 90% in 5% CO2 incubator. The stock solutions of pristine BNNT, P123, A.O, PEI, F127, P123-BNNT, A.O-BNNT, PEI-BNNT and F127-BNNT were serial diluted in MEM medium (1000, 500, 250, 125, 62.5, 31.25, 15.62, 7.8 μg mL−1). These samples were labelled as BNNT, P, A.O, PEI, F, P-BNNT, A.O-BNNT, PEI-BNNT and F-BNNT, respectively.
The modified samples with different concentrations were added to the wells and after 24 h of incubation the media was discarded again and was washed with MEM media. 200 μL of MTT (5 mg mL−1 of 1× PBS) reagent was added to each well after washing. Following 4–6 hours of incubation, 1 mL of DMSO was added and again left for incubation of 10 minutes and then the absorbance was read at 540 nm with a microplate reader. The cell viability (% of control) is expressed as (ODtest − ODblank)/(ODcontrol − OD blank) × 100, where ODtest is the optical density of the cells exposed to different samples, ODcontrol is the optical density of the cells untreated with samples and ODblank is the optical density of the wells without cells.39
2.6 Characterization Techntques
X-ray diffraction patterns of BNNTs were recorded on a PAN analytical X'Pert pro diffractometer analyzer, using a Cu Kα (λ = 1.54 Å) radiation source operated with 40 kV 230 mA, at a scan rate of (2θ) 0.02° per sec and from 10 to 80°. High resolution transmission electron microscopic images of BNNTs were obtained from JEOL-2100 instrument operated at 200 kV. In the analysis, the samples were ultrasonically dispersed in acetone and dropped onto a carbon-coated copper gird to analyse pristine BNNTs and in case of the suspended solutions a drop on copper grid was analysed. X-ray photoelectron spectra (XPS) were recorded on an Omicron Nanotechnology GMBH spectrometer employing monochromatic AlKα (1486.6 eV) X-ray source. The Epoch Microplate Spectrophotometer was used to measure the absorbance of cells in MTT assay method.2.7 DNA fragmentation assay
Qualitative analysis of DNA fragmentation was carried out by agarose gel electrophoresis. The typical procedure was followed as reported by Perreault et al. 2012.40 The cells treated with the samples were collected into tubes, washed with PBS and centrifuged. TE (10 mM Tris, 1 mM EDTA, pH 8.0) buffer was added to the remaining pellet. About 300 μL of phenol–chloroform–isoamyl alcohol (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added to DNA extract and again centrifuged. After aspirating the supernatant 500 μL of 70% ethanol was added to the pellet and again centrifuged, after discarding the supernatant the obtained pellet was finally air dried. TE buffer was added to it and about 20 μg mL−1 of DNA was separated by 1.5% agarose gel electrophoresis. The migration of the DNA was observed for 3 h at 70 V and visualised under ultraviolet light after staining with ethidium bromide.
1) was added to DNA extract and again centrifuged. After aspirating the supernatant 500 μL of 70% ethanol was added to the pellet and again centrifuged, after discarding the supernatant the obtained pellet was finally air dried. TE buffer was added to it and about 20 μg mL−1 of DNA was separated by 1.5% agarose gel electrophoresis. The migration of the DNA was observed for 3 h at 70 V and visualised under ultraviolet light after staining with ethidium bromide.
2.8 Fluorescence studies using acridine orange (AO) and ethidium bromide (EB)
Samples of BNNT, F, and F-BNNTs for their corresponding IC50 values were suspended in MEM media and incubated with MCF-7 and A549 cancer cells for 24 h. Similarly, the cancer cells MCF-7 and A549 cells were incubated with the tamoxifen loaded F-BNNTs (F-BNNT-Tam) and paclitaxel loaded F-BNNTs (F-BNNTs-Pax) for 24 h for their corresponding IC50 values. Following the incubation period, the cells were washed with PBS twice and 50 μL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of AO–EB were added to each plate and again incubated cells for 20 min for fluorescence imaging. The cells were again washed with 200 μL PBS and viewed under fluorescence microscopy using blue filter (510–590 nm) (Soares et al. 2012).41 Zeiss axiovert 200 M (Hg lamp) motorized inverted fluorescent microscope was used for fluorescence microscopic studies of cells with markers.
1 ratio of AO–EB were added to each plate and again incubated cells for 20 min for fluorescence imaging. The cells were again washed with 200 μL PBS and viewed under fluorescence microscopy using blue filter (510–590 nm) (Soares et al. 2012).41 Zeiss axiovert 200 M (Hg lamp) motorized inverted fluorescent microscope was used for fluorescence microscopic studies of cells with markers.
3. Results and discussions
3.1 Characterisation of synthesised boron nitride nanotubes
BNNTs obtained by the reaction of B/V2O5/Ni2O3 at 1100 °C were acid purified and characterised by XRD, HRTEM, Raman spectroscopy and XPS techniques depicted in Fig. 1a–g. The high peak intensities and well ordered BNNT structure with crystalline nature can be clearly seen in Fig. 1a. The purified pattern of BNNTs assigned with the peak values 26.61° (3.35 Å), 41.93° (2.16 Å), 54.62° (1.68 Å) and 76.17 (1.25 Å) corresponds to (002), (100), (004) and (110) diffractions peaks of hexagonal phase boron nitride and the calculated lattice constants a = 2.5 and c = 6.7 are consistent with the literature (JCPDS, file no. 85-1068 a = 2.510 and c = 6.690). The purified pattern suggests that BNNTs structures were not damaged by acid treatments and metal particles, either vanadium or nickel peaks were not identified.42 | ||
| Fig. 1 Purified BNNTs XRD pattern (a), HR-TEM images (b and c), Raman spectra (d), survey scan of BNNTs (e), individual XPS spectra of B1s and N1s (g and h). | ||
HRTEM lattice fringes images of BNNTs (Fig. 1b and c) demonstrates the morphology of multiwall boron nitride nanotubes (MWBNNT) with straight tubular in structure with inner diameter <10 nm and outer diameter around 25 nm. The images further confirm that the tube walls are not damaged with the acid treatment which underwent purification steps and no catalyst particles adhered in the tube surface or in the side walls. The images obtained for our BNNTs were similar to the reports of Zhi et al. 2005.43
The Raman spectra indicated a sharp peak with high intensity (Fig. 1d) centred at 1367 cm−1 attributed to the E2g mode of vibration where B and N atoms are moving against one another within a plane. The Raman spectra figured out in this study was similar to the results of Pakdel et al. 2012.44 The purity of BNNTs were further analysed with XPS technique which is shown in (Fig. 1e–g), the full survey scan gives the elemental composition of boron, carbon, nitrogen and oxygen present in the sample. The presences of carbon and oxygen spectrum in both the cases are due to the surface contamination during the exposure of the sample in the air before analysis (Fig. 1e). The major composition of boron and nitrogen was identified from the individual spectra of B1s and N1s. (Fig. 1f and g). These results confirmed the crystalline structure, morphology and purity of the samples.
3.2 Noncovalent functionalization and suspension of BNNTs in aqueous media
Many research groups have been reported on covalent and non-covalent functionalisation methods to solubilize BNNTs.26–29 In these functionalization, non-covalent approaches have several advantages over covalent since the nanotube structures and their properties can be preserved after the modification process (Velayudham et al. 2010).25 Using this method, the surface of BNNTs can be modified by different techniques, creating a suitable platform on the surface of these materials, enabling non-covalent attachment of polymeric groups to the surface of BNNTs. These polymer groups enhance their dispersity in aqueous media. The suspension of BNNTs in aqueous media is an important factor to excel its applications in biomedical field.The pictorial representation in the Fig. 2 shows the attachment of polymer groups P, A.O, PEI and F on the surface of BNNTs. These coatings enhance the suspension of BNNTs in aqueous media. In these polymer group P, PEI and F are non-ionic surfactant where A.O is an ionic surfactant. For the first time BNNTs suspension in aqueous media is examined with P and F polymers as shown in image Fig. 2a and b. Both the non ionic tri-block polymers has the structural composition of polyethylene oxide and polypropylene oxide [(HO(CH2CH2O)x(CH2CH(CH3)O)y(CH2CH2O)x)H]. The chain length is the main difference in the polymers if x = 20 and y = 70 it is P, where in case of F x = 100 and y = 65. Polyethylene oxide is hydrophilic in nature (tail group) and polypropylene oxide (head group) is hydrophobic. The ratio between hydrophilic to hydrophobic chain lengths determines the dispersity of BNNTs in aqueous solution. When the ratio between hydrophilic to hydrophobic groups is high, the suspension of polymeric solutions in water is high.45 BNNTs suspension in F is greater than P due its hydrophilic attraction towards surrounding water and interaction of BNNTs with water molecules that leads to stabilisation of BNNTs for long period of time.
 | ||
| Fig. 2 Pictorial representation of polymeric surfactants wrapped on BNNTs (a) P-BNNT, (b) F-BNNT, (c) PEI-BNNT and (d) A.O-BNNT. | ||
PEI bulk molecules wrapped on the surface of BNNTs leads to its fine dispersion in polar solvents (Fig. 2c). Ciofani et al. have reported on the BNNTs stable dispersion in PEI was due to the attachment of amino groups on the surface of the BNNTs and increases their interactions with the biological molecules.24 In AO-BNNTs, (Fig. 2d) hydrophile–lipophile groups of tails and heads permits the BNNTs to be suspended as the core micelles in the thick surfactant solutions. The long-term stabilisation of BNNTs and its interaction with the ionic surfactant is governed by the weak forces of attraction.37 The BNNTs stable suspension can be arranged in the order of F-BNNT > PEI-BNNT > P-BNNT > A.O-BNNT. The pristine BNNTs and polymer coated BNNTs suspensions in water were shown in Fig. 3.
 | ||
| Fig. 3 Pristine BNNT and surfactant modified BNNTs suspension in water (a) pristine BNNTs, (b) P-BNNTs, (c) A.O-BNNTs, (d) PEI-BNNTs and (d) F-BNNTs. | ||
The Initial weight loss in the range of 50–200 °C corresponds to the vaporization of moisture adsorbed in the sample.46,47 The weight loss from 200–400 °C attributed to the oxidation of BN particles.42 A slow increase in weight from 400–700 °C and very steep increase in sample weight above 800 °C was attributed to the oxidation of BNNTs and formation of B2O3.48,49 TGA pattern shown in Fig. 5 is similar to that of BNNTs obtained by ball milling method.50 From this result it is evident that the BNNTs synthesised by B/V2O5/Ni2O3 is thermally stable up to 700 °C in air.
Fig. 6 shows the TGA patterns of PEI-BNNTs, P-BNNTs, A.O-BNNTs and F-BNNTs. The thermal stability and the percentage of PEI, P, A.O and F surfactants coated on BNNTs were determined from the TGA patterns. For all the samples about 5 mg were used for TGA analysis in order to identity the specific amount of polymeric substance coated in BNNTs. All the coated samples show a weight loss in the range of 230–550 °C which is mainly due to the degradation of polymeric surfactants. For PEI-BNNTs, the significant weight loss of 7.540% (0.3600 mg) occurs at the temperature of 290–550 °C suggest the complete decomposable of PEI. From this weight loss it was inferred about 0.36 mg of PEI is coated on ∼5 mg of BNNTs. A slow increase in weight was observed above 900 °C is mainly attributed to the oxidation of BNNTs.
In the case of P-BNNTs, the maximum weight loss of 7.462% (0.35 mg) in the range of 325–358 °C corresponds to the complete decomposition of P123 polymer (Kao et al. 2005). Similar to PEI-BNNTs, in P-BNNTs the weight of the sample increases above 900 °C. For A.O-BNNTs, the weight loss of 10.79% (0.48 mg) at 300–373 °C is attributed to the complete elimination of A.O. The increase in the weight of AO-BNNTs was observed above 900 °C due to the effect of oxidation process. The weight loss of about 5.99% (0.38 mg) was found to be occurred in the range of 230–530 °C for F-BNNTs. This particular weight loss at this temperature was attributed to the complete degradation of F polymer (Yan et al. 2005). In this sample the increase in weight was occurred above 900 °C. From these observations we confirmed that all the coated BNNTs are thermally stable up to 900 °C and the percentage of PEI, P, A.O and F coated on MW-BNNTs was found to be 0.36 mg, 0.35 mg, 0.48 mg and 0.38 mg, respectively.
3.3 Antibacterial studies of BNNTs against E. coli and S. aureus
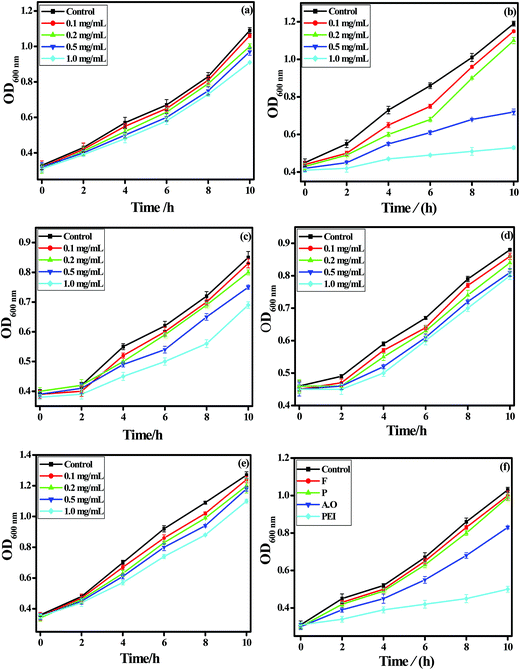
The antibacterial activity of pristine MW-BNNTs, coated MW-BNNTs and polymer solutions were investigated in Gram negative Escherichia coli (E. coli) and Gram positive Staphylococcus aureus (S. aureus) bacteria's. Fig. 7 and 8 shows the OD growth curves of E. coli and S. aureus after they were treated by pristine MW-BNNTs, coated MW-BNNTs and polymer solutions for a desired period of time.Fig. 7a–e shows the OD growth curves of treated E. coli cells by BNNTs, PEI-BNNTs, A.O-BNNTs, P-BNNTs and F-BNNTs at different concentrations (0.1, 0.2, 0.5 and 1.0 mg mL−1) for 1 h. BNNTs treated bacterial cells shows increase in the growth with regular period of time (Fig. 7a). The hydrophobic nature of BNNTs and weak van der Waals forces of attraction between OH group and BN are the prime reason for its non interaction with bacterial cells. This evidently suggests that BNNTs did not exhibit antibacterial activity against E. coli. In contrast, coated BNNTs exhibited good dispersion in water influenced by the polymer and ionic surfactant coatings on the surface nanotubes.
Among PEI, A.O, P and F coated BNNTs, PEI-BNNTs possess strong antibacterial activity against E. coli as observed from the Fig. 7b. At lower concentration the time required to attain the exponential growth phase is significantly different and no delays in the growth of bacterial cells was observed.38 When the concentration of PEI-BNNTs increased to 1.0 mg mL−1 there is no obvious growth within 10 h. This indicates that at higher concentration, PEI-BNNTs can almost inactive all the bacterial cells.
Noteworthy, the branched PEI molecules coated on the surface of BNNTs induces antibacterial activity against bacterial species. In general, PEI is a cationic amphiphilic polymer which can induce membrane disruption or permeabilization, leading to antibacterial activity. The positive charge in PEI neutralizes the negative charge on the bacterial cell membranes. Therefore, the selective permeability deactivates the outer cellular membrane and so, it possesses strong antibacterial activity.51,52 It was also reported that high molecular weight (50 kDa) of branched PEI exhibits multiple mechanisms in targeting the gram negative (E. coli) and as well as gram positive bacteria's (S. aureus).53
The E. coli cells treated by A.O-BNNTs show least antibacterial activity even at higher concentration (1.0 mg mL−1) compared to PEI-BNNTs (Fig. 7c). This indicates that the growth of bacterial cells increases with increase in time in lower and higher concentrations. A.O is an ionic surfactant and attracts the BNNTs with weak van der Waals forces of attraction that leads to the dispersibility of BNNTs in aqueous media. The permeability factor of A.O-BNNTs towards the cell membrane is very less. Hence, the bulk molecules anchored on the surface of BNNTs inactivated the cells neither by permeabilization nor by disruption. P123-BNNTs and F127-BNNTs possess no inherent antibacterial activity and the cell growth increases with increase in time (Fig. 7d and e). The delay in the growth was not observed in both the polymers even at higher concentration. Moreover, P123 and F127 both are neutral polymers which have hydrophobic and hydrophilic chain groups, which will not inactivate the cells but helps BNNTs to be well dispersed in aqueous media.
Fig. 7f compares the OD growth curves of E. coli after they have treated by polymer solution F, P, A.O and PEI at a concentration of 0.5 mg mL−1. Among the polymer solutions, PEI exhibits strong antibacterial activity and inactivate E. coli. The other surfactants F and P have no inherent antibacterial activity towards the bacterial cells. The ionic surfactant A.O exhibits least activity against bacterial cells. These results confirm that the uncoated BNNTs did not exhibit any significant antibacterial activity to bacterial cells. The non-covalent modification via surfactant molecules improve only the dispersion of BNNTs but not induces antibacterial activity like PEI functionalized BNNTs.
Similar results were also observed in case of S. aureus cells treated by BNNTs, PEI-BNNTs, A.O-BNNTs, P-BNNTs and F-BNNTs for 1 h (Fig. 8a–e). The BNNT treated S. aureus culture shows gradual increase in growth with regular period of time at lower and higher concentrations (Fig. 8a). The uncoated BNNTs did not exhibit any significant antibacterial activity to bacterial cells. It should be pointed out among coated BNNTs; PEI-BNNTs inhibit the growth of S. aureus within 10 h of higher concentrations and increase in the growth at lower concentration (Fig. 8b). This is due to the time required to deactivate the growth of bacterial cells at lower concentration is different than in higher concentration.
A.O-BNNT possesses very minimal antibacterial activity at higher concentration (Fig. 8c). In case of P-BNNT and F-BNNT also the inhibitory action against S. aureus is extremely low (Fig. 8d and e). A comparison of Fig. 8c–e with 8a demonstrates that the delay in growth time of AO-BNNTs, P-BNNTs and F-BNNTs is shorter than by the PEI-BNNTs under the same concentrations. This suggests that the antibacterial activity of PEI-BNNTs is stronger than that of AO-BNNTs, P-BNNTs and F-BNNTs. This is confirmed by the decrease in OD values of PEI-BNNTs treated S. aureus cells compared to the OD values of AO-BNNTs, P-BNNTs and F-BNNTs at different concentration.
Fig. 8f compares the OD growth curves of S. aureus after they are treated by polymer solution F, P, A.O and PEI at a concentration of 0.5 mg mL−1. Similar to that of E. coli cells, in S. aureus also PEI possess strong antibacterial activity and inhibits the growth of micro-organism with respect to time. A.O exhibits very low inhibitory action where as P and F surfactants did not exhibit any antibacterial activity compared to that of PEI.
Finally, these results suggest that BNNTs (pristine) has no inherent antibacterial activity against E. coli and S. aureus. Among coated BNNTs, PEI-BNNTs exhibit strong antibacterial activity towards E. coli and S. aureus cultures. In surfactants, PEI exhibits strong antibacterial activity. These results reveal that, the bacterial cell death was merely due to the influence of PEI in coated BNNTs. These samples were further examined by TEM technique.
 | ||
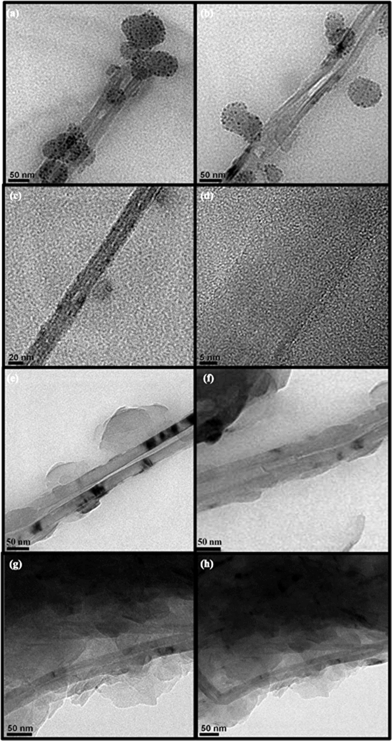
| Fig. 9 TEM images of E. coli and S. aureus (a and b), BNNT treated E. coli (c and d) and BNNT treated S. aureus (e and f) samples. | ||
CNTs, structurally similar to BNNT possess antibacterial activity. It was reported that pristine SWCNTs in direct contact with E. coli exhibit strong antimicrobial activity and can cause severe membrane damage E. coli cells.54 In contrast, even though BNNTs aggregates with the E. coli and S. aureus, the membrane damage or inactivation of cells are not observed from the TEM images.
Fig. 10a and b clearly shows the TEM images of PEI-BNNT and free PEI treated E. coli cells. It should be mentioned that, in both these images BNNTs did not damage the cell membrane but PEI diffusion causes cell damage. On other hand complete denaturation can be visualized from the Fig. 10c due to the influence of PEI alone, this evidently suggests that it causes severe damages to E. coli cells. Similar observations were noticed from the TEM images PEI-BNNTs and free PEI treated S. aureus (Fig. 11a–c). So, this study confirmed that BNNTs are non-toxic to micro-organisms but PEI coated BNNTs causes damage to E. coli and S. aureus cells.
 | ||
| Fig. 10 TEM images of PEI-BNNT treated E. coli cells (a and b) and free PEI treated E. coli cells (c). | ||
 | ||
| Fig. 11 TEM images of PEI-BNNT treated S. aureus cells (a and b) and free PEI treated S. aureus cells (c). | ||
These results suggested that nanograms coatings of PEI with BNNTs might be used as antibacterial agents in food systems and medicine for inhibiting certain pathogens. The PEI coated BNNTs can be used as ultrafiltration membranes in water purification process and in food packing systems.55
3.4 Cytotoxicity studies on normal and tumor cells
 | ||
| Fig. 13 Cytotoxicity results on Chang liver cells: MTT assay after 24 h incubation pristine BNNTs, surfactants (a) and coated BNNTs (b). | ||
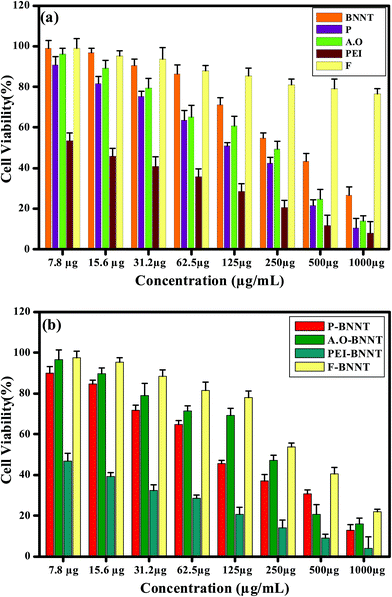
The cytotoxicity levels of BNNT, P, A.O, PEI and F determined by MTT assay after 24 h of incubation with Vero cells are shown in Fig. 12a. It was observed that 98.8% of cells are found to be viable after 24 h of incubation with 7.8 μg mL−1 concentration of BNNTs. At 1000 μg mL−1 concentration only 22.2% of cells are found to be viable. The reduction in the percentage of viable cells from 98.8 to 50.18 (IC50) is noticed at 250 μg mL−1 concentration and above this concentration BNNTs are found to be toxic. It was also reported that pristine BNNTs are found to be toxic to MRC-5 (human lung fibroblast cells), MCF-7 (adenocarcinoma mammary gland cells), T98 (mutant p53) and U87 (wild-type p53) cells above 200 μg mL−1 concentration.32 From this result it was suggested that BNNTs are nontoxic in nature at lower concentration and at higher concentration it induce toxicity to cells. In P polymer treated Vero cells, the percentage of viability decreased from 98.4 to 17.45 at concentration 7.8 and 1000 μg mL−1, respectively. Similarly, in case of A.O the cell viability decreased from 93.79% to 19.37% at concentrations 7.8 and 1000 μg mL−1, respectively. The IC50 value for both the surfactants was observed at 250 μg mL−1 concentration.
For PEI treated Vero cells at 7.8 μg mL−1 of concentration 62% of cells are viable and at 1000 μg mL−1 of concentration only 5.42% of cells are viable. It was inferred from MTT result that PEI can inhibit the 50% of cells at a very low (15.6 μg mL−1) concentration. In case of F polymer maximum cell viability of 95.07% and 72.48% was maintained at initial (7.8 μg mL−1) and final (1000 μg mL−1) concentrations, respectively. Comparing the four polymer solutions F treated Vero cells exhibit good viability and the biocompatibility nature of surfactants can be in the order of F > A.O > P > PEI.
Fig. 12b represents the MTT assay results of Vero cells incubated for 24 h with surfactant coated BNNTs. The cell viability of 90.69%, 97.69%, 48.52% and 99.22% was observed at the initial concentrations (7.8 μg mL−1) of P-BNNTs, A.O-BNNTs, PEI-BNNTs and F-BNNTs respectively. Their cell viability was reduced to 41.16%, 46.3%, and 55.78% at 250 μg mL−1 concentration for P-BNNTs, A.O-BNNTs and F-BNNTs, respectively. At final concentration (1000 μg mL−1) 20.15%, 24.41% and 35.63% cells are viable. In PEI-BNNTs, the 50% cell reduction was observed at initial (7.8 μg mL−1) concentration and at final concentration (1000 μg mL−1) only 4.41% of cells are viable. These results suggest PEI-BNNTs are cytotoxic in nature compared to P-BNNTs, A.O-BNNTs and F-BNNTs. Ciofani et al. reported that BNNTs suspensions in PEI are extremely stable but its toxic nature was due to PEI permeabilization into plasma membranes and disruption of endosome, as well as lysosome complexes.24 It was also reported that PEI was toxic to HeP-2 cells and its dosage needs to be limited to short term tropical treatment rather than system administration for long term infection treatment.53 In this work, the MTT test also confirmed the toxic nature of PEI and its concentration should to be limited to nanograms. Based on the MTT results the cytocompatability behaviour of coated BNNTs can be arranged in the order of F-BNNT > A.O-BNNT > P-BNNT > PEI-BNNT.
Similar experiments were also carried out on Chang liver cells to confirm the biocompatible nature of pristine BNNTs, coated BNNTs and surfactants. Fig. 13a depicts the percentage of cell viability of Chang liver cells after 24 h of incubation with BNNT, P, A.O, PEI, and F at different concentration. It was observed that 7.8 μg mL−1 of BNNTs treated Chang liver cells retains 99.1% of cells after 24 h of incubation and 54.57% (IC50) of cells are viable at 250 μg mL−1 which is similar to that of Vero cells. For P surfactant treated Chang liver cells, 50% of cells are viable at 125 μg mL−1 concentrations. The reduction in cell viability was due to the cytostatic action of P (P123) polymer. In case of A.O treated cells, the IC50 value was observed at 250 μg mL−1 concentration. In PEI treated Chang liver cells, only 53.5% of cells are viable at initial concentration. Surprisingly, for F polymer treated BNNTs 76.43% of cells are in control at 1000 μg mL−1 concentration.
Fig. 13b shows the percentage of cell viability of Chang liver cells after 24 h of incubation with coated BNNTs at different concentration. For P-BNNTs, A.O-BNNTs, PEI-BNNTs and F-BNNTs the IC50 values (45.5%, 47%, 46.9% and 53.6%) are attained at the concentrations of 125 μg mL−1, 250 μg mL−1, 7.8 μg mL−1 and 250 μg mL−1 respectively. In Vero and Chang liver cells the overall MTT results confirm that pristine BNNTs are nontoxic in nature below 250 μg mL−1 concentration. Among the surfactants, F polymer is nontoxic compared to A.O, P and PEI. In coated BNNTs, F-BNNTs are considered to nontoxic than P-BNNTs, A.O-BNNTs and PEI-BNNTs. In both the cells the extensive cytocompatabile nature of F solutions are due to the hydrophilic side chains of PEO which prevents from aggregation, protein adsorption and recognition by the reticuloendithelial systems.
In general, pluronic polymers are non degradable in nature due to its high molecular weight, but it gets filtered by the kidney and cleared in urine.58 Among the non-ionic polymers P and F solutions, F exhibits more cell viability than P, due to the difference in their hydrophilic (PEO) units. Even though same structural units of [(PEO)x(PPO)y(PEO)x] are present, the number of x molecules determines it's hydrophilic to hydrophobic nature.45 US food and drug administration (FDA) approved the usage of pluronic solutions in humans with certain molecular weights for oral or intravenous administration.59 A.O is an ionic surfactant, which exhibits cytocompatability. It is widely used for drug delivery as it is also easily eliminated by kidney. In both the cells, pristine BNNTs, F solutions and F-BNNTs, exhibited good cytocompatability after 24 h of incubation. The moderate cytocompatability was observed in P solutions, P-BNNTs, A.O solutions and A.O-BNNTs. PEI and PEI-BNNTs were found to be toxic at lower concentrations.
Fig. 14 represents the MTT assay results of MCF-7 cells and A549 cells after culturing for 24 h. The MCF-7 cells treated with pristine BNNTs, F-BNNTs, F-BNNTs loaded tamoxifen (F-BNNT-Tam) and free tamoxifen are illustrated in Fig. 14a. For BNNTs, the cell viability decreases from 98.8% to 25.3% at 7.8 to 1000 μg mL−1 concentrations. At 125 μg mL−1 and 250 μg mL−1 concentrations, 14.0% and 50% of cell death was observed. In F-BNNTs, cell viability decreases from 99.2% to 26.48% at 7.8 to 1000 μg mL−1 concentrations. The 50% of cell death was observed at 250 μg mL−1 concentration for BNNTs and F-BNNTs in normal cells and cancer cells. These results suggest that BNNT and F-BNNT are not toxic to cells at lower concentration but it might have important toxicity at higher concentration (>250 μg mL−1).
 | ||
| Fig. 14 MTT assay results of BNNT, F-BNNTs, tamoxifen, paclitaxel and F-BNNTs-Tam/Pax interactions with MCF-7 (a) and A-549 cells (b) after 24 h of incubation. | ||
In case of free tamoxifen at lower (7.8 μg mL−1) concentration, 14.73% of cell reduction was observed. Further increase in concentration of tamoxifen markedly decreased the cell viability to 77.52% at 1000 μg mL−1. At 125 μg mL−1 of concentration, 50% of growth inhibition was observed after 24 h of the culture. In contrast, F-BNNT-Tam strongly inhibits the growth of MCF-7 cells at lower concentration. At initial 7.8 μg mL−1 concentration, 33.8% of cell death was observed. As the concentration of F-BNNT-Tam increases, 90.6% of cell death was observed at final concentration (1000 μg mL−1). The 50% cell reduction was observed at 31.2 μg mL−1 concentration. These results suggest that F-BNNTs are good carrier of drug.
Gelmann reported that high dose of tamoxifen decreases the therapeutic index by increasing toxicity.60 The enhancement in growth inhibition of MCF-7 cells is due to incorporation of drug and it is independent of composition on F-BNNTs. There are mainly two possible processes that account for the toxicity of MCF-7 cells. First process is the release of tamoxifen from F-BNNTs outside the cells followed by increase in the cytotoxicity of tamoxifen by nanotubes. Second one is the release of drug from inside the cell and creates toxicity to the cells.61
Fig. 14b shows the cytotoxicity results of Pristine BNNTs, F-BNNT, paclitaxel and paclitaxel loaded F-BNNTs (F-BNNT-Pax) treated A549 cells after 24 h of incubation. For BNNTs, the cell viability decreases from 98.4% to 29.5% at 7.8 to 1000 μg mL−1 concentrations respectively. At 125 and 250 μg mL−1 concentration cell death of 19.9% and 50% was observed respectively. In F-BNNTs, cell viability decreases from 99.1% to 31.63% at 7.8 to 1000 μg mL−1 and the 50% of cell death was observed at 250 μg mL−1 concentrations. Similar to MCF-7, A549 cells also exhibit the toxicity of BNNTs in the range of 125–250 μg mL−1 concentration. Noteworthy in the case of normal cells, similar concentration of BNNTs induce toxicity and it is also reflected in the case of F-BNNTs. From these results it was further suggested that BNNTs might be toxic to cells at higher concentration.
For paclitaxel treated A549 cells, at lower (7.8 μg mL−1) concentration, 19.8% of cell reduction was observed. Further increase in concentration of paclitaxel decreased the cell viability to 86.8% at 1000 μg mL−1. In the case of F-BNNT-Pax, the cell death of at 34.79% and 95.6% was observed at initial (7.8 μg mL−1) and final concentration (1000 μg mL−1) respectively. The 50% of growth inhibition was observed at 15.6 μg mL−1 concentration. Compared to free paclitaxel in F-BNNTs loaded paclitaxel, the reduction in cell viability occurred at lower concentration. The in vitro delivery of drugs tamoxifen and paclitaxel attached to F-BNNTs exhibited higher efficiency in suppressing tumor growth than delivery of drug alone. In addition, BNNTs have the capacity for the selective adsorption of neutron which can be used for killing cancer cells alone.
 | ||
| Fig. 15 MTT assay results of tamoxifen and paclitaxel interactions with Vero cells after 24 h of incubation. | ||
In the case of paclitaxel treated Vero cells, 10.86% and 89.15% of cell reduction was observed at 7.8 and 1000 μg mL−1 concentration respectively. Paclitaxel inhibits 19.8% and 86.8% of A549 cells at the same concentrations of Vero cells. From the MTT results it was inferred that anticancer drugs (tamoxifen and paclitaxel) have toxicity to degrade the Vero cells as that of MCF7 and A549 cells. It was reported that the chemotherapeutic drugs (tamoxifen and paclitaxel) create serious side effects and also affect the growth of normal human cells. Particularly, Brian et al. reported the adverse effects of tamoxifen on breast cells can induce antiestrogenic and proestrogenic problems in endometrium and bone.62 In addition, tamoxifen is also associated in creating thromboembolic disease and stroke. Liew et al. reported that the periodical dosage of paclitaxel to patients resulted in fatal pneumonia and esophagus disorders.63 These results suggest that the drug dosage should to be limited to short term and there is an unmet need to develop cell-targeting drug formulations with a wide therapeutic index. BNNTs have shown great promise as nanoscaled vehicles for targeted drug delivery. The in vitro delivery of drugs tamoxifen and paclitaxel attached to F-BNNTs showed higher efficiency in suppressing tumor growth than delivery of drug alone. In case of F-BNNTs, at initial (7.8 μg mL−1) concentration only 0.8% of cell reduction was observed in Vero cells after 24 h of incubation which is comparatively very lower than free tamoxifen and paclitaxel. Approximately 70% of cells are in control at 125 μg mL−1 concentration. Similar results were observed in MCF7 and A549 cells. These results revealed the biocompatible nature of F-BNNTs and its use in targeted drug delivery.
Pristine BNNTs at their IC50 concentration (250 μg mL−1) show DNA laddering which indicates that it can induce apoptosis to the cells (Lane 1). In control MCF7 cells no laddering was appeared and DNA is found to be intact (Lane 2). F-BNNTs, at their IC50 (250 μg mL−1) concentration shows DNA laddering (Lane 3). Also, in case of F solution, DNA laddering was absent and clearly indicates that F polymer even at higher concentration (>500 μg mL−1) did not fragment the cells (Lane 4). The formation of fragmentation in F-BNNTs occurs due to the influence of BNNTs alone. In addition, F-BNNTs-Tam shows DNA laddering (Lane 5).
F-BNNT-Pax depicted the DNA laddering for A549 treated cells (Lane 6). Pristine BNNTs shows DNA laddering as observed in case of MCF7 cells (Lane 7). F polymer did not show DNA laddering (Lane 8). F-BNNTs confirm DNA laddering in A549 cells as that of MCF7 cells (Lane 9). Lane 10 corresponds to the control sample of A549 cell. The inhibitory action of the BNNTs, F-BNNTs, F-BNNTs-Tam and F-BNNTs-Pax on the MCF-7 and A549 cells was due to apoptosis. The samples which shows DNA fragmentation are due to the activation of caspase cascades, which cleaves the specific substrates that is responsible for DNA repair activation.64
Fig. 17a and b shows the untreated MCF7 cells stained by AO/EB exhibits the homogenous green fluorescence confirming the healthy cells with nucleus and cytoplasm. On other hand MCF7 cells treated with BNNT, F polymer, F-BNNT and F-BNNT-Tam treated for 24 h at their respective IC50 250, 1000, 250, 31.2 and 15.6 μg mL−1 concentrations are shown in Fig. 17c–j. The bright field images of these samples are demonstrated in the Fig. 17 a, c, e, g and i, respectively. After treating cells with IC50 concentration of the BNNTs for 24 h, we have observed cytological changes like late orange apoptotic cells to red fluorescing nuclei (Fig. 17d) with condensed or fragmented chromatin. In F polymer, even at 1000 μg mL−1 the cells are quiet viable. On other hand F-BNNTs are found to be cytotoxic to cells at its IC50 (250 μg mL−1) concentration (Fig. 17h). This confirms the toxic nature of BNNTs at this concentration induces cytotoxic to cells. An F-BNNTs-Tam treated cell expresses (Fig. 17j) cytological changes in cells at a concentration of 31.2 (μg mL−1) which is very lower than the cytotoxicity concentration of BNNTs and F-BNNTs. These results evidence that tamoxifen induce the cell death through apoptosis. We therefore conclude that F-BNNTs-Tam exhibits stronger cytotoxic effect to MCF7 cells.
Fig. 18a and b represent the microscopic images of untreated viable A549 cells exhibiting the homogenous green fluorescence of healthy cells with nucleus and cytoplasm. The phase contrast images of BNNTs, F polymer, F-BNNTs and F-BNNT-Pax treated cells illustrate the morphological changes as shown in the Fig. 18c, e, g and i, respectively. These cells are treated with their respective IC50 concentration as mentioned in case of MCF7 cells. BNNTs treated A549 cells shows difference in the shapes and cell shrinkage characteristics of apoptotic cell death. Moreover, chromatin condensation and DNA fragmentation are also observed in the BNNTs treated cells at its IC50 (250 μg mL−1) concentration.
In MC7 and A549 cells BNNTs are considered to be cytotoxic at higher concentration. In F polymer the viable cells exhibits green fluorescence while in F-BNNTs apoptotic cells show orange to red fluorescence (Fig. 18f and h). F-BNNTs-Pax treated cells and their morphological changes can be observed in Fig. 18j.
The result confirms that BNNTs induce apoptosis at 250 μg mL−1 concentration, and below that, the cytotoxicity level is maintained. Also, in F-BNNTs at the same concentration apoptosis occurs due to the influence of BNNTs. The cancer drugs paclitaxel and tamoxifen are widely used in the patients after the radiation therapy and this drug is administrated up to a particular dosage intravenously. Paclitaxel induces side effects such as bronchodilation, angioedema, hypertension and tamoxifen. Apart from this also some other serious side effects such as vision problems, unusual bleeding, swelling of eyes, face, tongue, throat muscle weakness, etc., also occur. F-BNNTs loaded with these drugs at lower concentration will reduce these side effects by targeting the particular cells, but the optimisation of these results with different dose in normal cells and tumour cells must be examined by in vivo.
4. Conclusions
In summary, pristine BNNTs, coated BNNTs antibacterial activity and cytotoxicity was evaluated by different in vitro methods. BNNTs coated with P123, PEI, F-127 and ammonium oleate shows strong dispersion in aqueous media. Pristine BNNTs have no antibacterial activity while PEI and PEI-BNNTs exhibits strong antibacterial activity against E. coli and S. aureus. Pristine BNNTs, F127 and F-BNNTs hardly expresses good biocompatibility with Vero, Chang liver (normal) and MCF-7, A549 (cancer) cells up to 250 μg mL−1. P-BNNTs and A.O-BNNTs were found to be cytocompatible with Vero, Chang liver, MCF-7 and A549 cells up to 125 μg mL−1. PEI-BNNTs were found to be toxic to the Vero and Chang liver cells at lower concentrations. F-BNNT-Tam and F-BNNTs-Pax induced apoptosis to MCF-7 and A549 cells at lower concentrations (31.2 and 15.6 μg mL−1). These results obtained in this study were considered essential for biomedical application and further in vivo studies could definitely extend the application in cancer diagnosis and treatment.References
- G. Oberdorster, J. Intern. Med., 2010, 267, 89–105 CrossRef CAS PubMed
.
- S. Y. Madani, N. Naderi, O. Dissanayake, A. Tan and A. M. Seifalian, Int. J. Nanomed., 2012, 7, 905–914 CAS
.
- D. Golberg, Y. Bando, C. C. Tang and C. Zhi, Adv. Mater., 2007, 19, 2413–2432 CrossRef CAS
.
- S. Kalay, Z. Yilmaz and M. Culha, Beilstein J. Nanotechnol., 2013, 4, 843–851 CrossRef CAS PubMed
.
- L. Hu, D. Hecht and G. Gruner, Chem. Rev., 2010, 110, 5790 CrossRef CAS PubMed
.
- J. Wang, C. H. Lee and Y. K. Yap, Nanoscale, 2010, 2, 2028–2034 RSC
.
- C. H. Lee, M. Xie, V. Kayastha, J. Wang and Y. K. Yap, Chem. Mater., 2010, 22, 1782–1787 CrossRef CAS
.
- J. Li, H. Lin, Y. Chena, Q. Sua and Q. Huanga, Chem.–Eng. J., 2011, 174, 687–692 CrossRef CAS PubMed
.
- D. Ozmen, N. A. Sezgi and S. Balci, Chem.–Eng. J., 2013, 219, 28–36 CrossRef CAS PubMed
.
- L. Li, X. Liu, L. Li and Y. Chen, Microelectron. Eng., 2013, 110, 256–259 CrossRef CAS PubMed
.
- L. L. Sartinska, A. A. Frolov, A. F. Andreeva, A. M. Kasumov, I. I. Timofeeva and M. I. Danilenko, Mater. Lett., 2011, 65, 1791–1793 CrossRef CAS PubMed
.
- D. Seo, J. Kim, S. H. Park, Y. U. Jeong, Y. S. Seo, S. H. Lee and J. Kim, J. Ind. Eng. Chem., 2013, 19, 1117–1122 CrossRef CAS PubMed
.
- S. Jain, S. R. Singh and S. Pillai, J. Nanomed. Nanotechol., 2012, 3, 1000140 Search PubMed
.
- C. Zhi, Y. Bando, C. Tang, S. Honda, K. Sato, H. Kuwahara and D. Golberg, Angew. Chem., Int. Ed., 2005, 44, 7932–7935 CrossRef CAS PubMed
.
- S.-Y. Xie, W. Wang, K. A. S. Fernando, X. Wang, Y. Lin and Y.-P. Sun, Chem. Commun., 2005, 3670–3672 RSC
.
- T. Ikuno, T. Sainsbury, D. Okawa, J. M. J. Freechet and A. Zettl, Solid State Commun., 2007, 42, 643–646 CrossRef PubMed
.
- G. Ciofani, G. G. Genchi, I. Liakos, A. Athanassiou, D. Dinucci, F. Chiellini and M. Virgilio, J. Colloid Interface Sci., 2012, 374, 308–314 CrossRef CAS PubMed
.
- S.-J. Zhou, C.-Y. Ma, Y.-Y. Meng, H.-F. Su, Z. Zhu, S.-L. Deng and S.-Y. Xie, Nanotechnology, 2012, 23, 055708 CrossRef PubMed
.
- C. Y. Zhi, Y. Bando, T. Terao, C. C. Tang, H. Kuwahara and D. Golberg, Chem.–Asian J., 2009, 4, 1536–1540 CrossRef CAS PubMed
.
- C. Y. Zhi, Y. Bando, C. C. Tang and D. Golberg, J. Am. Chem. Soc., 2005, 127, 17144–17145 CrossRef CAS PubMed
.
- C. Y. Zhi, Y. Bando, W. L. Wang, C. C. Tang, H. Kuwahara and D. Golberg, Chem.–Asian J., 2007, 2, 1581–1585 CrossRef CAS PubMed
.
- P. Wu, X. Chen, N. Hu, U. C. Tam, O. Blixt, A. Zettl and C. R. Bertozzi, Angew. Chem., Int. Ed. Engl., 2008, 47, 5022–5025 CrossRef CAS PubMed
.
- X. Chen, P. Wu, M. Rousseas, D. Okawa, Z. Gartner, A. Zettl and C. R. Bertozzi, J. Am. Chem. Soc., 2009, 131, 890–891 CrossRef CAS PubMed
.
- G. Ciofani, V. Raffa, A. Menciassi and A. Cuschieri, Biotechnol. Bioeng., 2008, 101, 850–858 CrossRef CAS PubMed
.
- S. Velayudham, C. H. Lee, M. Xie, D. Blair, N. Bauman, Y. K. Yap, S. A. Green and H. Liu, ACS Appl. Mater. Interfaces, 2010, 2, 104–110 CAS
.
- G. Ciofani, L. Ricotti, S. Danti, S. Moscato, C. Nesti, D. Alessandro, D. Dinucci, F. Chiellini, A. Pietrabissa, M. Petrini and A. Menciassi, Int. J. Nanomed., 2010, 5, 285–298 CrossRef CAS
.
- G. Ciofani, S. Danti, D. Alessandro, L. Ricotti, S. Moscato, G. Bertoni, A. Falqui, S. Berrettini, M. Petrini, V. Mattoli and A. Menciassi, ACS Nano, 2010, 4, 6267–6277 CrossRef CAS PubMed
.
- J.-H. Choi, J. Kim, D. Seo and Y.-S. Seo, Mater. Res. Bull., 2013, 48, 1197–1203 CrossRef CAS PubMed
.
- Y.-T. R. Lau, M. Yamaguchi, X. Li, Y. Bando, D. Golberg and F. M. Winnik, J. Phys. Chem. C, 2013, 117, 19568–19576 CAS
.
- G. Ciofani, V. Raffa, A. Menciassi and A. Cuschieri, Nanoscale Res. Lett., 2009, 4, 113–121 CrossRef CAS PubMed
.
- D. Lahiri, F. Rouzaud, T. Richard, A. K. Keshri, S. R. Bakshi, L. Kos and A. Agarwal, Acta Biomater., 2010, 6, 3524–3353 CrossRef CAS PubMed
.
- T. H. Ferreira, P. R. O. Silva, R. G. Santos and E. M. B. Sousa, J. Biomater. Nanobiotechnol., 2011, 2, 426–434 CrossRef CAS
.
- G. Ciofani, S. Danti, L. Ricotti, D. Alessandro, S. Moscato, S. Berrettini, V. Mattoli and A. Menciassi, Curr. Nanosci., 2011, 7, 94–109 CrossRef CAS
.
- G. Ciofani, S. Danti, G. G. Genchi, D. Alessandro, J.-L. Pellequer, M. Odorico, V. Mattoli and M. Giorgi, Int. J. Nanomed., 2012, 7, 19–24 CrossRef CAS PubMed
.
- J. S. M. Nithya and A. Pandurangan, RSC Adv., 2014, 4, 26697–26705 RSC
.
- T. Oku, N. Koi, K. Suganuma, V. B. Rodion and Y. Kawazoe, Solid State Commun., 2007, 143, 331–336 CrossRef CAS PubMed
.
- J. Yu, Y. Chen and B. M. Cheng, Solid State Commun., 2009, 149, 763–766 CrossRef CAS PubMed
.
- Y. Bai, I. S. Park, S. J. Lee, T. S. Bae, F. Watari, M. Uo and M. H. Lee, Carbon, 2011, 49, 3663–3671 CrossRef CAS PubMed
.
- L. Zhang, F. Jiang, Y. Chen, J. Luo, S. Liu, B. Zhang, Z. Ye, W. Wang, X. Liang and W. Shi, Int. J. Mol. Sci., 2013, 12, 24742–24754 CrossRef PubMed
.
- F. Perreault, S. P. Melegari, C. H. Costa, A. L. D.-O. F. Rossetto, R. Popovic and W. G. Matias, Sci. Total Environ., 2012, 441, 117–124 CrossRef CAS PubMed
.
- M. A. Soares, J. A. Lessa, I. C. Mendes, J. G. D. Silva, R. G. Santos, L. B. Salum, H. Daghestani, A. D. Andricopulo, B. W. Day, A. Vogt, J. L. Pesquero, W. R. Rocha and H. Beraldo, Bioorg. Med. Chem., 2012, 20, 3396–3409 CrossRef CAS PubMed
.
- H. Chen, Y. Chen, J. Yu and J. S. Williams, Chem. Phys. Lett., 2006, 425, 315–319 CrossRef CAS PubMed
.
- C. Zhi, Y. Bando, C. Tan and D. Golberg, Solid State Commun., 2005, 135, 67–70 CrossRef CAS PubMed
.
- A. Pakdel, C. Zhi, Y. Bando, T. Nakayama and D. Golberg, Nanotechnology, 2012, 23, 21560 CrossRef PubMed
.
- Z. Wu, C. Guo, S. Liang, H. Zhang, L. Wang, H. Sunc and B. Yanga, J. Mater. Chem., 2012, 22, 18596–18602 RSC
.
- L. T. Podobeda, A. K. Tsapuk and A. D. Buravov, Sov. Powder Metall Met. Ceram., 1976, 165, 44–47 Search PubMed
.
- T. Matsuda, J. Mater. Sci., 1989, 24, 2353–2358 CrossRef CAS
.
- Y. Chen, J. Zou, S. J. Campbell and G.-L. Caer, Appl. Phys. Lett., 2004, 84, 2430–2432 CrossRef CAS PubMed
.
- Y. Song, Y. Sun, D. H. Shin, K. N. Yun, Y.-H. Song, W. I. Milne and C. J. Lee, Appl. Phys. Lett., 2014, 104, 163102–163105 CrossRef PubMed
.
- Y. Chen, J. F. Gerald, J. S. Williams and S. Bulcock, Chem. Phys. Lett., 1999, 3–4, 260–264 CrossRef
.
- H. S. Saier Jr, Enzymes in Metabolic Pathways: A Comparative Study of Mechanism, Structure, Evolution, and Control, Harper & Row Publishers, New York, 1987, pp. 48–59 Search PubMed
.
- D. L. Jack, N. M. Yang and M. H. Saier Jr, Eur. J. Biochem., 2001, 268, 3620–3639 CrossRef CAS
.
- K. A. Gibney, I. Sovadinova, A. I. Lopez, M. Urban, Z. Ridgway, G. A. Caputo and K. Kuroda, Macromol. Biosci., 2012, 12, 1279–1289 CrossRef CAS PubMed
.
- S. kang, M. S. Mauter and M. limelech, Langmuir, 2007, 23, 8670–8673 CrossRef CAS PubMed
.
- L. Yu, Y. Zhang, B. Zhang, J. Liu, H. Zhang and C. Song, J. Membr. Sci., 2013, 447, 452–462 CrossRef CAS PubMed
.
- N. S. Rejinolda, A. Naira, M. Sabithaa, K. P. Chennazhia, H. Tamurab, S. V. Naira and R. Jayakumara, Carbohydr. Polym., 2012, 87, 943–949 CrossRef PubMed
.
- M. Senevirathne, Y.-J. Jeon, Y.-T. Kim, P.-J. Park, W.-K. Jung, C.-B. Ahn and J.-Y. Je, Carbohydr. Polym., 2012, 87, 876–880 CrossRef CAS PubMed
.
- W. Zhang, Y. Shi, Y. Chen, J. Ye, X. Sha and Fang, Biomaterials, 2011, 32, 2894–2906 CrossRef CAS PubMed
.
- J. Sobczynski, S. Kristensen and K. Berg, Photochem. Photobiol. Sci., 2014, 13, 8–22 CAS
.
- E. P. Gelmann, J. Natl. Cancer Inst., 1996, 88, 224–226 CrossRef CAS PubMed
.
- H. L. Wong, A. M. Rauth, R. Bendayan, J. L. Manias, M. Ramaswamy, Z. Liu, S. Z. Erhan and X. Y. Wu, Pharm. Res., 2006, 23, 1574–1584 CrossRef CAS PubMed
.
- R. J. H. B. Brian and M. S. Sandra, Clin. Cancer Res., 2008, 14, 14–24 CrossRef PubMed
.
- M. S. Liew, J. Sia, M. H. W. Starmans, A. Tafreshi, S. Harris, M. Feigen, S. White, A. Zimet, P. Lambin, P. C. Boutros, P. Mitchell and T. John, Cancer Med., 2013, 6, 916–924 CrossRef PubMed
.
- M. Jeyaraj, M. Rajesh, R. Arun, D. MubarakAli, G. Sathishkumar, G. Sivanandhan, G. Kapil Dev, M. Manickavasagam, K. Premkumar, N. Thajuddin and A. Ganapathi, Colloids Surf., B, 2013, 102, 708–717 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2014 |