Synthesis of benzil derivatives via oxidation of alkynes catalyzed by Pd–Fe3O4 heterodimer nanocrystals†
Abstract
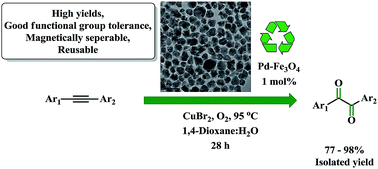
An efficient, iterative, catalytic, Wacker-type oxidation of alkynes to 1,2-diketones using a Pd–Fe3O4 heterodimer nanocrystalline catalyst has been developed. This process has a wide substrate scope and affords 1,2-diketo compounds in excellent yields under atmospheric conditions. The operational procedure using the Pd–Fe3O4 nanocatalyst is extremely easy, and the catalyst can be recovered by employing simple magnetic separation, enabling the recycling of the catalyst five times without loss of catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...