Organocatalytic direct asymmetric vinylogous Mannich reaction of γ-butenolides with isatin-derived ketimines†
Yunlong Guoab,
Yong Zhangab,
Liangwen Qiab,
Fang Tiana and
Lixin Wang*a
aKey Laboratory of Asymmetric Synthesis and Chirotechnology of Sichuan Province, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu 610041, People's Republic of China. E-mail: wlxioc@cioc.ac.cn; Fax: +86-028-8525-5208
bUniversity of Chinese Academy of Sciences, Beijing 10039, People's Republic of China
First published on 11th June 2014
Abstract
The first direct asymmetric vinylogous Mannich reaction of γ-butenolides with isatin-derived ketimines has been effectively realized promoted by a bifunctional quinidine-derived catalyst. A series of chiral 3-aminooxindole derivatives bearing adjacent tertiary and quaternary stereocenters with the butenolide moiety were obtained in excellent yields (up to 97%) and high enantioselectivities (up to 96% ee).
Vinylogous addition1 is one of the most important reactions for the direct formation of C–C bonds. Metal complexes2 and organocatalysts3 promoted asymmetric vinylogous Mannich reactions of aldimines affording optically active δ-amino-α,β-unsaturated carbonyl compounds have been reported in the past decades. For the lower reactivity of ketimines compared with aldimines, the catalytic asymmetric vinylogous Mannich reaction of ketimines4 is still rare. A few examples of enantioselective vinylogous Mannich reaction of ketimines catalyzed by transition metal catalysts have been documented. The first example of the addition reaction between ketimines and siloxyfurans catalyzed by a chiral phosphine–AgOAc complex was revealed by Hoveyda and co-workers.5a Recently, Nakamura and co-workers5b reported an enantioselective vinylogous Mannich reaction of siloxyfurans with N-phosphinoyl ketimines promoted by Cu(OAc)2 and cinchona alkaloid amide complex. Subsequently, Shibasaki5c disclosed a direct catalytic asymmetric vinylogous Mannich reactions of γ-butenolides with N-thiophosphinoyl ketimines in the presence of a soft Lewis acid [Cu(CH3CN4)]PF6–(R,RP)-TANIAPHOS complex and a hard Brønsted base. Except for those above cases, a more straightforward and atom-economical vinylogous Mannich reaction of ketimines used as electrophilic component is still highly desirable. To the best of our knowledge, the small molecular catalytic direct asymmetric vinylogous Mannich reaction of ketimines has not been reported.
3-Aminooxindole, as a core structure, widely exists in a variety of bioactive molecules.6 Over the past years, a number of efforts have been made to enantioselective syntheses of those architectures.7,8 In 2012, Wang8b reported the Mannich reaction of N-alkoxycarbonyl ketimines derived from isatins with 1,3-dicarbonyl compounds catalyzed by tertiary amine thioureas, in which N-alkoxycarbonyl ketimines showed extremely high reactivity. Based on those backgrounds, we envisioned that the asymmetric direct vinylogous Mannich reaction of γ-butenolides and N-alkoxycarbonyl ketimines may be realized by bifunctional organocatalyst and afforded enantioenriched 3-aminooxindoles with multifunctional groups. We recognized that the tertiary amine group would activate γ-butenolide and the hydroxyl group would activate N-alkoxycarbonyl ketimine through hydrogen bondings. Thus the synergistic interactions would ensure high stereoselectivity in this transformation, affording the corresponding optically active products via a postulated transition state (TS) as shown in Fig. 1. For further enriching the methodology for the preparations of chiral 3-aminooxindole subunits and expanding our research on asymmetric syntheses of optical oxindole derivatives.9 Herein, we wish to present the first direct organocatalytic asymmetric vinylogous Mannich reaction of γ-butenolides10 and N-Boc ketimines derived from isatins (Fig. 2).
Initially, ketimine 1a and γ-butenolide 2a were chosen as substrates and carried out at 30 °C in DCM. A variety of organocatalysts were tested and the results were summarized in Table 1. In all cases, the addition proceeded smoothly while with poor enantioselectivities (Table 1, entries 1–5, 0–24% ee), when thiourea and squaramide catalysts derived from cinchona alkaloids were screened. Fairly good enantioselectivity (Table 1, entry 8, 64% ee) was observed in the presence of cinchonine 4h. The enantioselectivity was further improved to 74% ee (Table 1, entry 10) when quinine derived catalyst 4j was added. Finally, quinidine derived catalyst 4k gave the desired product with the best result (Table 1, entry 11, 85% ee) and was selected as the optimal catalyst.
| Entry | Catalysts | t (h) | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|
| a Reactions were carried out with 1a (0.1 mmol), 2a (0.2 mmol) and catalyst 4 (10 mol%) in DCM (0.5 mL) at 30 °C.b Isolated yield.c Determined by HPLC analysis.d The ee value was determined by chiral HPLC analysis. | |||||
| 1 | 4a | 53 | 81 | 75/25 | 2 |
| 2 | 4b | 47 | 90 | 76/24 | 6 |
| 3 | 4c | 53 | 85 | 77/23 | 0 |
| 4 | 4d | 27 | 96 | 76/24 | 24 |
| 5 | 4e | 48 | 75 | 78/22 | 3 |
| 6 | 4f | 27 | 77 | 78/22 | 27 |
| 7 | 4g | 27 | 85 | 79/21 | 51 |
| 8 | 4h | 27 | 83 | 80/20 | 64 |
| 9 | 4i | 27 | 78 | 80/20 | 59 |
| 10 | 4j | 27 | 83 | 80/20 | 74 |
| 11 | 4k | 24 | 90 | 79/21 | 85 |
| 12 | 4l | 12 | 85 | 76/24 | 80 |
| 13 | 4m | 12 | 94 | 75/25 | 84 |
In the presence of catalyst 4k, the N-substituted groups, temperature, catalyst loading and solvent were then screened and the results were summarized in Table 2. The results showed that the N-substituted groups had slight effect on enantioselectivity (Table 2, entries 1–3) and N-Bn ketimine gave better enantioselectivity (88% ee, Table 2, entry 3). Lowering the temperature resulted in an increase of enantioselectivity (Table 2, entries 4–6) and the product was obtained with 94% ee at −30 °C (Table 2, entry 6). Lowering the catalyst loading, better enantioselectivity (Table 2, entry 7, 96% ee) was obtained in the presence of 5 mol % of 4k. Solvents slightly affected the enantioselectivity (Table 2, entries 7–12) and DCM was selected as the optimal solvent.
| Entry | R | Solvent | t (h) | yieldb (%) | drc | eed |
|---|---|---|---|---|---|---|
| a Reactions were carried out with 1 (0.1 mmol), 2a (0.2 mmol) and catalyst 4k (10 mol%) in solvent (0.5 mL).b Isolated yield.c Determined by HPLC analysis.d The ee value was determined by chiral HPLC analysis.e At −10 °C.f At −20 °C.g At −30 °C.h 5 mol% of 4k was used. | ||||||
| 1 | Allyl | DCM | 12 | 97 | 65/35 | 87 |
| 2 | Boc | DCM | 18 | 81 | 76/24 | 82 |
| 3 | Bn | DCM | 13 | 96 | 67/33 | 88 |
| 4e | Bn | DCM | 12 | 95 | 62/38 | 92 |
| 5f | Bn | DCM | 12 | 93 | 62/38 | 92 |
| 6g | Bn | DCM | 12 | 96 | 64/36 | 94 |
| 7g,h | Bn | DCM | 14 | 94 | 67/33 | 96 |
| 8g,h | Bn | CHCl3 | 36 | 93 | 61/39 | 94 |
| 9g,h | Bn | DCE | 13 | 95 | 59/41 | 92 |
| 10g,h | Bn | Toluene | 26 | 91 | 58/42 | 94 |
| 11g,h | Bn | THF | 36 | 47 | 44/56 | 96 |
| 12g,h | Bn | CH3CN | 36 | 72 | 58/42 | 86 |
Under the optimal reaction conditions, the substrate scope of the reaction was further explored to test the generality of this protocol (Table 3). Generally, good yields, moderate diastereoselectivities and high enantioselectivities were obtained for various substituents on the aromatic ring of ketimines 1 (Table 3, entries 1–14, 56–97% yield, 50/50–79/21 dr, 83–96% ee). N-substituted groups were also examined and good results were obtained (Table 3, entries 1–4, 66–97% yields, 68/32–79/21 dr, 83–94% ee). When 4-Br ketimine 1o was used, no product was detected (Table 3, entry 15), maybe for its larger steric hindrance. The optimal protocol was also expanded to 3,4-dibromofuran-2(5H)-one 2b with 20 mol% 4k as catalyst, and moderate to good yields, moderate diastereoselectivities and high enantioselectivities (Table 3, entries 16–26, 60–95% yield, 59/41–68/32 dr, 87–93% ee) were obtained.
| Entry | 1 | 2 | 3 | t (h) | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|---|---|
| a Reactions were carried out with 1 (0.1 mmol), 2 (0.2 mmol) and catalyst 4k in DCM (0.5 mL) at −30 °C.b Isolated yield.c Determined by HPLC analysis.d The ee value was determined by chiral HPLC analysis.e At room temperature.f 20 mol% of 4k was used. | |||||||
| 1 | 1a | 2a | 3aa | 15 | 95 | 77/23 | 90 |
| 2 | 1b | 2a | 3ba | 17 | 94 | 79/21 | 91 |
| 3 | 1c | 2a | 3ca | 17 | 97 | 68/32 | 94 |
| 4 | 1d | 2a | 3da | 39 | 66 | 75/25 | 83 |
| 5 | 1e | 2a | 3ea | 14 | 94 | 67/33 | 96 |
| 6 | 1f | 2a | 3fa | 25 | 69 | 58/42 | 90 |
| 7 | 1g | 2a | 3ga | 16 | 77 | 50/50 | 91 |
| 8 | 1h | 2a | 3ha | 24 | 87 | 61/39 | 88 |
| 9 | 1i | 2a | 3ia | 24 | 77 | 60/40 | 90 |
| 10 | 1j | 2a | 3ja | 36 | 95 | 65/35 | 92 |
| 11 | 1k | 2a | 3ka | 44 | 93 | 64/36 | 91 |
| 12 | 1l | 2a | 3la | 48 | 70 | 63/37 | 90 |
| 13 | 1m | 2a | 3ma | 15 | 56 | 59/41 | 91 |
| 14e | 1n | 2a | 3na | 48 | 92 | 65/35 | 88 |
| 15 | 1o | 2a | 3oa | 216 | NR | — | — |
| 16f | 1b | 2b | 3bb | 22 | 93 | 59/41 | 90 |
| 17f | 1c | 2b | 3cb | 36 | 95 | 68/32 | 92 |
| 18f | 1e | 2b | 3eb | 90 | 95 | 65/35 | 91 |
| 19f | 1f | 2b | 3fb | 209 | 59 | 60/40 | 90 |
| 20f | 1g | 2b | 3gb | 216 | 60 | 64/36 | 92 |
| 21f | 1h | 2b | 3hb | 216 | 64 | 64/36 | 89 |
| 22f | 1i | 2b | 3ib | 209 | 65 | 63/37 | 87 |
| 23f | 1j | 2b | 3jb | 209 | 82 | 66/34 | 91 |
| 24f | 1k | 2b | 3kb | 36 | 96 | 60/40 | 91 |
| 25f | 1l | 2b | 3lb | 36 | 71 | 60/40 | 93 |
| 26f | 1p | 2b | 3pb | 209 | 95 | 66/34 | 88 |
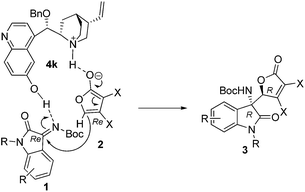
A plausible mechanism was proposed for the direct asymmetric Mannich reaction (Fig. 3). The substrates were activated by the bifunctional catalyst. γ-Butenolide was activated by the tertiary amine group and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond of ketimine was activated by hydroxyl group of catalyst. To avoid steric repulsion between the R moiety of ketimine and the X substituent of dienolate, the Re face of dienolate attacked the Re face of C
N double bond of ketimine was activated by hydroxyl group of catalyst. To avoid steric repulsion between the R moiety of ketimine and the X substituent of dienolate, the Re face of dienolate attacked the Re face of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond to form the product. The absolute configuration was determined by an X-ray analysis of the single crystal of 3aa, which was assigned as (R, R).11
N double bond to form the product. The absolute configuration was determined by an X-ray analysis of the single crystal of 3aa, which was assigned as (R, R).11
Conclusions
In conclusion, we have first developed a highly effective organocatalytic direct vinylogous Mannich reaction of isatin-derived ketimine with γ-butenolide. A series of 3-aminooxindoles bearing adjacent quaternary and tertiary stereocenters were obtained in good yields and excellent enantioselectivities.Notes and references
- For selected reviews: (a) G. Casiraghi, L. Battistini, C. Curti, G. Rassu and F. Zanardi, Chem. Rev., 2011, 111, 3076 CrossRef CAS PubMed; (b) S. E. Denmark, J. R. Heemstra, Jr and G. L. Beutner, Angew. Chem., Int. Ed., 2005, 44, 4682 CrossRef CAS PubMed; (c) S. V. Pansare and E. K. Paul, Chem.–Eur. J., 2011, 17, 8770 CrossRef CAS PubMed; (d) S. F. Martin, Acc. Chem. Res., 2002, 35, 895 CrossRef CAS PubMed; (e) S. Bur and S. F. Martin, Tetrahedron, 2001, 57, 3221 CrossRef CAS.
- For selected examples: (a) S. F. Martin and O. D. Lopez, Tetrahedron Lett., 1999, 40, 8949 CrossRef CAS; (b) E. L. Carswell, M. L. Snapper and A. H. Hoveyda, Angew. Chem., Int. Ed., 2006, 45, 7230 CrossRef CAS PubMed; (c) A. Yamaguchi, S. Matsunaga and M. Shibasaki, Org. Lett., 2008, 10, 2319 CrossRef CAS PubMed; (d) H. Mandai, K. Mandai, M. L. Snapper and A. H. Hoveyda, J. Am. Chem. Soc., 2008, 130, 17961 CrossRef CAS PubMed; (e) A. S. González, R. G. Arrayás, M. R. Rivero and J. C. Carretero, Org. Lett., 2008, 10, 4335 CrossRef PubMed; (f) Z.-L. Yuan, J.-J. Jiang and M. Shi, Tetrahedron, 2009, 65, 6001 CrossRef CAS PubMed; (g) H.-P. Deng, Y. Wei and M. Shi, Adv. Synth. Catal., 2009, 351, 2897 CrossRef CAS; (h) Q.-Y. Zhao, Z.-L. Yuan and M. Shi, Adv. Synth. Catal., 2011, 353, 637 CrossRef CAS; (i) Q.-Y. Zhao and M. Shi, Tetrahedron, 2011, 67, 3724 CrossRef CAS PubMed; (j) L. Zhou, L.-L. Lin, J. Ji, M.-S. Xie, X.-H. Liu and X.-M. Feng, Org. Lett., 2011, 10, 3056 CrossRef PubMed.
- For selected examples: (a) D. Uraguchi, K. Sorimachi and M. Terada, J. Am. Chem. Soc., 2004, 126, 11804 CrossRef CAS PubMed; (b) M. Sickert and C. Schneider, Angew. Chem., Int. Ed., 2008, 47, 3631 CrossRef CAS PubMed; (c) D. S. Giera, M. Sickert and C. Schneider, Org. Lett., 2008, 10, 4259 CrossRef CAS PubMed; (d) T. Akiyama, Y. Honma, J. Itoh and K. Fuchibe, Adv. Synth. Catal., 2008, 350, 399 CrossRef CAS; (e) M. Sickert, F. Abels, M. Lang, J. Sieler, C. Birkemeyer and C. Schneider, Chem.–Eur. J., 2010, 16, 2806 CrossRef CAS PubMed; (f) Y.-L. Guo, J.-F. Bai, L. Peng, L.-L. Wang, L.-N. Jia, X.-Y. Luo, F. Tian, X.-Y. Xu and L.-X. Wang, J. Org. Chem., 2012, 77, 8338 CrossRef CAS PubMed.
- For selected reviews: (a) M. Shibasaki and M. Kanai, Chem. Rev., 2008, 108, 2853 CrossRef CAS PubMed; (b) O. Riant and J. Hannedouche, Org. Biomol. Chem., 2007, 5, 873 RSC; (c) B. Karimi, D. Enders and E. Jafari, Synthesis, 2013, 45, 2769 CrossRef CAS PubMed; for selected examples: (d) N. Hara, R. Tamura, Y. Funahashi and S. Nakamura, Org. Lett., 2011, 13, 1662 CrossRef CAS PubMed; (e) R. Shintani, M. Takeda, Y.-T. Soh, T. Ito and T. Hayashi, Org. Lett., 2011, 13, 2977 CrossRef CAS PubMed; (f) T. Kano, S.-H. Song, Y. Kubota and K. Maruoka, Angew. Chem., Int. Ed., 2012, 51, 1191 CrossRef CAS PubMed; (g) F.-G. Zhang, H. Ma, J. Nie, Y. Zheng, Q.-Z. Gao and J.-A. Ma, Adv. Synth. Catal., 2012, 354, 1422 CrossRef CAS; (h) H.-N. Yuan, S. Wang, J. Nie, W. Meng, Q.-W. Yao and J.-A. Ma, Angew. Chem., Int. Ed., 2013, 52, 3869 CrossRef CAS PubMed; (i) L. Yin, Y.-M. Bao, N. Kumagai and M. Shibasaki, J. Am. Chem. Soc., 2013, 135, 10338 CrossRef CAS PubMed; (j) M. G. Núňez, A. J. M. F. Farley and D. J. Dixon, J. Am. Chem. Soc., 2013, 135, 16348 CrossRef PubMed.
- (a) L. C. Wieland, E. M. Vieira, M. L. Snapper and A. H. Hoveyda, J. Am. Chem. Soc., 2009, 131, 570 CrossRef CAS PubMed; (b) M. Hayashi, M. Sano, Y. Funahashi and S. Nakamura, Angew. Chem., Int. Ed., 2013, 52, 5557 CrossRef CAS PubMed; (c) L. Yin, H. Takada, N. Kumagai and M. Shibasaki, Angew. Chem., Int. Ed., 2013, 52, 7310 CrossRef CAS PubMed.
- For selected reviews: (a) A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945 CrossRef CAS PubMed; (b) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef CAS PubMed; (c) F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381 CrossRef CAS; (d) J. E. M. N. Klein and R. J. K. Taylor, Eur. J. Org. Chem., 2011, 6821 CrossRef CAS.
- For selected reviews and examples: (a) R. Dalpozzo, G. Bartoli and G. Bencivenni, Chem. Soc. Rev., 2012, 41, 7247 RSC; (b) K. Shen, X.-H. Liu, L.-L. Lin and X.-M. Feng, Chem. Sci., 2012, 3, 327 RSC; (c) B.-D. Cui, W.-Y. Han, Z.-J. Wu, X.-M. Zhang and W.-C. Yuan, J. Org. Chem., 2013, 78, 8833 CrossRef CAS PubMed; (d) Y.-Q. Zhang, Y.-A. Yuan, G.-S. Liu and H. Xu, Org. Lett., 2013, 15, 3910 CrossRef CAS PubMed; (e) L. Ren, X.-L. Lian and L.-Z. Gong, Chem.–Eur. J., 2013, 19, 3315 CrossRef CAS PubMed; (f) F.-L. Hu, Y. Wei, M. Shi, S. Pindic and G.-G. Li, Org. Biomol. Chem., 2013, 11, 1921 RSC.
- (a) Q.-X. Guo, Y.-W. Liu, X.-C. Li, L.-Z. Zhong and Y.-G. Peng, J. Org. Chem., 2012, 77, 3589 CrossRef CAS PubMed; (b) W.-J. Yan, D. Wang, J.-C. Feng, P. Li, D.-P. Zhao and R. Wang, Org. Lett., 2012, 14, 2512 CrossRef CAS PubMed; (c) J.-C. Feng, W.-J. Yan, D. Wang, P. Li, Q.-T. Sun and R. Wang, Chem. Commun., 2012, 48, 8003 RSC; (d) N. Hara, S. Nakamura, M. Sano, R. Tamura, Y. Funahashi and N. Shibata, Chem.–Eur. J., 2012, 18, 9276 CrossRef CAS PubMed; (e) H. Lv, B. Tiwari, J.-M. Mo, C. Xing and Y.-G. Chi, Org. Lett., 2012, 14, 5412 CrossRef CAS PubMed; (f) D. Wang, J.-Y. Liang, J.-C. Feng, K.-R. Wang, Q.-T. Sun, L. Zhao, D. Li, W.-J. Yan and R. Wang, Adv. Synth. Catal., 2013, 355, 548 CAS; (g) Y.-L. Liu and J. Zhou, Chem. Commun., 2013, 49, 4421 RSC; (h) X.-J. Chen, H. Chen, X. Ji, H.-L. Jiang, Z.-J. Yao and H. Liu, Org. Lett., 2013, 15, 1846 CrossRef CAS PubMed; (i) S. Nakamura, K. Hyodo, M. Nakamura, D. Nakane and H. Masuda, Chem.–Eur. J., 2013, 19, 7304 CrossRef CAS PubMed; (j) T.-Z. Li, X.-B. Wang, F. Sha and X.-Y. Wu, Tetrahedron, 2013, 69, 7314 CrossRef CAS PubMed; (k) X.-B. Wang, T.-Z. Li, F. Sha and X.-Y. Wu, Eur. J. Org. Chem., 2014, 739 CrossRef CAS.
- (a) Q.-L. Wang, L. Peng, F.-Y. Wang, M.-L. Zhang, L.-N. Jia, F. Tian, X.-Y. Xu and L.-X. Wang, Chem. Commun., 2013, 49, 9422 RSC; (b) L.-L. Wang, J.-F. Bai, L. Peng, L.-W. Qi, L.-N. Jia, Y.-L. Guo, X.-Y. Luo, X.-Y. Xu and L.-X. Wang, Chem. Commun., 2012, 48, 5175 RSC; (c) L.-L. Wang, L. Peng, J.-F. Bai, L.-N. Jia, X.-Y. Luo, Q.-C. Huang, X.-Y. Xu and L.-X. Wang, Chem. Commun., 2011, 47, 5593 RSC; (d) L.-L. Wang, L. Peng, J.-F. Bai, Q.-C. Huang, X.-Y. Xu and L.-X. Wang, Chem. Commun., 2010, 46, 8064 RSC.
- For selected examples on vinylogous aldol reaction of furanones: (a) K. D. Sarma, J. Zhang and T. T. Curran, J. Org. Chem., 2007, 72, 3311 CrossRef PubMed; (b) H. Ube, N. Shimada and T. Terada, Angew. Chem., Int. Ed., 2010, 49, 1858 CrossRef CAS PubMed; (c) J. Luo, H.-F. Wang, X. Han, L.-W. Xu, J. Kwiatkowski, K.-W. Hang and Y.-X. Lu, Angew. Chem., Int. Ed., 2011, 50, 1861 CrossRef CAS PubMed; (d) Y. Yang, K. Zhang, J.-N. Zhao, J. Shi, L.-L. Lin, X.-H. Liu and X.-M. Feng, J. Org. Chem., 2012, 75, 5382 CrossRef PubMed; (e) S. V. Pansare and E. K. Paul, Chem. Commun., 2011, 47, 1027 RSC; (f) A. Claraz, S. Oudeyer and V. Levacher, Adv. Synth. Catal., 2013, 355, 841 CrossRef CAS; selected vinylogous Michael addition of furanones: (g) B. M. Trost and J. Hitce, J. Am. Chem. Soc., 2009, 131, 4572 CrossRef CAS PubMed; (h) H.-C. Huang, F. Yu, Z.-C. Jin, W.-J. Li, W.-B. Wu, X.-M. Liang and J.-X. Ye, Chem. Commun., 2010, 46, 5957 RSC; (i) J.-F. Wang, C. Qi, Z.-M. Ge, T.-M. Cheng and R.-T. Li, Chem. Commun., 2010, 46, 2124 RSC; (j) M. Terada and K. Ando, Org. Lett., 2011, 13, 2026 CrossRef CAS PubMed; (k) L. Yin, H. Takasa, S.-Q. Lin, N. Kumagai and M. Shibasaki, Angew. Chem., Int. Ed., 2013, 53, 5327 CrossRef PubMed.
- ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 985942. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra04824e |
| This journal is © The Royal Society of Chemistry 2014 |