SnO nanoparticles as an efficient catalyst for the one-pot synthesis of chromeno[2,3-b]pyridines and 2-amino-3,5-dicyano-6-sulfanyl pyridines†
Javad Safaei-Ghomi*,
Hossein Shahbazi-Alavi and
Elham Heidari-Baghbahadorani
Department of Organic Chemistry, Faculty of Chemistry, University of Kashan, P.O. Box 87317-51167, Kashan, Islamic Republic of Iran. E-mail: safaei@kashanu.ac.ir; Fax: +98-361-5912397; Tel: +98-361-5912385
First published on 2nd October 2014
Abstract
SnO nanoparticles have been used as an efficient catalyst for the preparation of chromeno[2,3-b]pyridines and 2-amino-3,5-dicyano-6-sulfanyl pyridines under reflux conditions in ethanol in good to excellent yields. This flexible and nano-catalytic procedure showed good recyclability and provides a clean condensation reaction in a short reaction time.
1. Introduction
The pyridine ring system is a structural component of a large number of biologically active compounds. Heterocycles with the pyridine moiety exhibit various pharmacological activities such as anti-bacterial,1 anticancer,2 antiprion3 potassium channel opening,4 IKK-binhibitors,5 potent inhibitor of HIV-1 integrase.6 Among the pyridine derivatives, 2-amino-3,5-dicyano-6-sulfanyl pyridines exhibit a broad range of biological properties such as treatment of the Hepatitis B Foundation infections,7 Creutzfeldt–Jacob disease, Parkinson's disease, hypoxia/ischemia, asthma, kidney disease, epilepsy and cancer.8–10 Condensed pyridines are known for their versatile biological activities, for example chromeno[2,3-b]pyridines have a number of pharmacological properties. Chromenopyridines are fused heterocyclic compounds that exhibit antibacterial (including antitubercular),11 anti-proliferative (cytotoxic activity against the human solid tumor HT-29 cell lines),12 antirheumatic,13 cancer chemopreventive and chemother-apeutic agent,14 antimyopic,15 antihistaminic,16 and antiasthmatic17 activities. Several compounds derived from these libraries were identified to inhibit mitogen-activated protein kinase-activated protein kinase 2 (MK-2) and suppress the expression of TNFα in U937 cells18 and possess gastric antisecretory activity in rats and dogs.19 Some other examples of chromenopyridine derivatives are the prominent drug molecules mlexanox (trade name Aphthasol), an antiallergic and topical antiulcer agent, and prosnoprofen (as NSAIDs), that have been reported (Fig. 1).Synthesis of bioactive compounds should be facile, flexible, rapid and practical in organic synthesis. Therefore, looking for efficient and concise methods for the synthesis of pyridines is an interesting challenge. Multi-component reactions (MCRs) present a wide range of possibilities for molecular diversity per step with a minimum of synthetic time, outlay, labour and waste production.20–25 Chromeno[2,3-b]pyridines show interesting characteristics which make them attractive targets for the synthesis via MCRs. Consequently, MCRs have been paid much attention by synthetic organic chemists worldwide.
Similarly nanoparticles have undergone extensive examination in the past decade. Due to their insolubility in reaction solvents renders them easily separable from the reaction mixture. Heterogeneous catalysis offers several advantages over the homogeneous counterpart, such as easy recovery, simple recycling without losing their activity and enhanced stability of the catalyst which can make synthetic processes cheaper, safer and greener.26–32 Therefore, the possibility of performing multicomponent reactions under mild conditions with a heterogeneous catalyst could enhance their effectiveness from economic and ecological points of view.
We wish to report herein a highly efficient procedure for the preparation of chromeno[2,3-b]pyridines and 2-amino-3,5-dicyano-6-sulfanyl pyridines using SnO nanoparticles as an efficient and reusable heterogeneous catalyst under reflux conditions in ethanol (Scheme 1). A number of methods have been developed for the synthesis of chromeno[2,3-b]pyridines and 2-amino-3,5-dicyano-6-sulfanyl pyridines using MCRs in the presence of few catalysts such as Et3N (under reflux conditions in ethanol),33 K2CO3 (EtOH:H2O, reflux).34
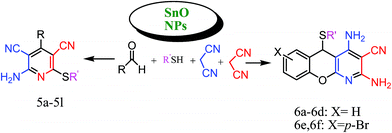 | ||
| Scheme 1 Synthesis of chromeno[2,3-b]pyridines and 2-amino-3,5-dicyano-6-sulfanyl pyridines using SnO nanoparticles. | ||
2. Results and discussion
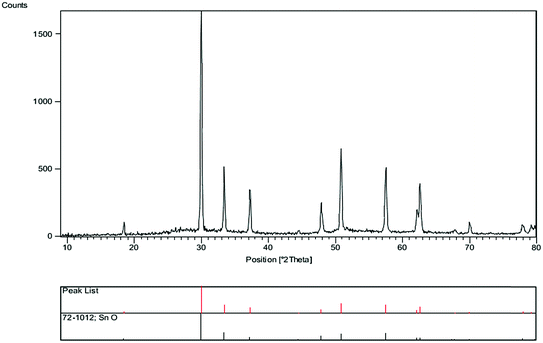
The first step entails the synthesis of highly stable SnO nanoparticles. The catalyst was prepared by co-precipitation technique and microwave radiation. For more investigation of the influence of microwave irradiation in this reaction, the synthesis of SnO nanoparticle was compared in two times. The precipitate dispersed in water kept under irradiation of microwave for 18 min and 12 min (Microwave power was maintained at 600 W). Particle size of SnO nanoparticles, was investigated by XRD pattern. The crystallite size diameter (D) of the SnO nanoparticles has been calculated by Debye–Scherrer equation (D = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), where FWHM (full-width at half-maximum or half-width) is in radians and θ is the position of the maximum of diffraction peak, K is the so-called shape factor, which usually takes a value about 0.9, and λ is the X-ray wavelength. Crystallite size of SnO has been found to be 28 nm for 18 min and 40 nm for 12 min. The XRD pattern of the SnO nanoparticles was shown in Fig. 2 (under irradiation of microwave for 18 min). The results show that SnO nanoparticles were obtained with an average diameter of 26–28 nm as confirmed by XRD analysis. The morphology and particle size of SnO nanoparticles, was investigated by scanning electron microscopy (SEM) (Fig. 3). The SEM images show particles with diameters in the range of nanometers.
θ), where FWHM (full-width at half-maximum or half-width) is in radians and θ is the position of the maximum of diffraction peak, K is the so-called shape factor, which usually takes a value about 0.9, and λ is the X-ray wavelength. Crystallite size of SnO has been found to be 28 nm for 18 min and 40 nm for 12 min. The XRD pattern of the SnO nanoparticles was shown in Fig. 2 (under irradiation of microwave for 18 min). The results show that SnO nanoparticles were obtained with an average diameter of 26–28 nm as confirmed by XRD analysis. The morphology and particle size of SnO nanoparticles, was investigated by scanning electron microscopy (SEM) (Fig. 3). The SEM images show particles with diameters in the range of nanometers.
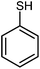
Initially, we had explored and optimized different reaction parameters for the synthesis of 2-amino-3,5-dicyano-6-sulfanyl pyridines by the condensation reaction of 4-nitrobenzaldehyde (1 mmol), 2.2 mmol of malononitrile and 1 mmol of thiophenol as a model reaction. Meanwhile, we investigated the reaction of salicylaldehydes, thiols and 2 equiv. of malononitrile in the presence of different catalysts for the synthesis of chromeno[2,3-b]pyridines. As given in Table 2, the solvent has a great effect on the acceleration of the reactions. Several reactions were scrutinized using various solvents such as EtOH, CH3CN, water, DMF and CHCl3. The best results were obtained under reflux conditions in ethanol and found that the reaction gave satisfying results in the presence of SnO nanoparticles at 6 mol% which gave excellent yields of products (Tables 1 and 2). The polarity, dipole moment, polarizability and hydrogen bonding of a solvent determine what type of compounds it is able to dissolve. In this reaction, the use of polar solvents favours the reaction mechanism. The catalyst showed best activity in ethanol compared to other organic solvents such as DMF, CH3CN, and CHCl3. The dielectric constant of solvents was listed in Table 2. The ethanol with dielectric constant 24.5 carried out reactions in good to excellent yields. Therefore, the use of polar solvents with moderate dielectric constant favours the condensation reactions. Also, the activity and stability of the SnO nanoparticles and those metal oxides in ethanol is maximum compared to other solvents. The model reactions was carried out in the presence of various catalysts such as metal oxide (MgO, CaO, CuO, ZrO2, SnO) and InCl3, CuCl, SnCl2 and CuSO4. When the reaction was carried out using CuO, MgO and SnO NPs as the catalyst, the product could be obtained in moderate to good yield. The acidity or basicity of those metal oxides or salts can be determined with the help of a very weak basic molecule which is only adsorbed on Lewis centres very weakly. The surface of a metal oxide consists of ordered arrays of acid–base centres. The cationic metal centres act as Lewis acid sites while the anionic oxygen centres act as Lewis bases. The strong solid acids or bases usually give higher activities in condensation reactions. We propose that SnO NPs have highly effective catalytic behaviour because of the surface properties that include Sn2+ as acid and O2− as base that accelerate the reaction rate. These complementary interactions between reactants and catalyst lead to an increase in the rate of reaction. Size of prepared SnO nanoparticles in the presence of microwave was reduced until 28 nm, and specific surface area was increased about 35 m2 g−1. Table 3 shows that the influence of particles size on the activity of the SnO nanoparticles in the synthesis of 6-sulfanyl pyridines and chromenopyridines. As expected, the increased surface area due to small particle size increased reactivity of catalyst. This factor is responsible for the accessibility of the substrate molecules on the catalyst surface. However, the activity of solid catalysts is influenced by the acid–base properties and many other factors such as geometric structure (particularly pore structure), the distribution of sites and the polarity of the surface sites.28
| Entry | Catalyst | (mol%) | Yieldsc 5a/6a (%) |
|---|---|---|---|
| a 1 mmol of 4-nitrobenzaldehyde, 2.2 mmol of malononitrile and 1 mmol of thiophenol.b Salicyladehyde (1.5 mmol), malononitrile (3 mmol) and benzenethiol (1.5 mmol).c Isolated yields. | |||
| 1 | — | — | 20/17 |
| 2 | MgO | 15 | 55/44 |
| 3 | CuCl | 10 | 42/32 |
| 4 | CuO | 10 | 40/22 |
| 5 | InCl3 | 5 | 24/27 |
| 6 | ZrO2 | 10 | 49/33 |
| 7 | CaO | 5 | 54/38 |
| 8 | CuSO4 | 10 | 30/18 |
| 9 | SnCl2 | 10 | 40/35 |
| 10 | SnO NPs | 3 | 82/79 |
| 11 | SnO NPs | 6 | 92/88 |
| 12 | SnO NPs | 9 | 92/89 |
| Entry | Solvent | Dielectric constant | Time 5a/6a (min) | Yieldsc 5a/6a (%) |
|---|---|---|---|---|
| a 1 mmol of 4-nitrobenzaldehyde, 2.2 mmol of malononitrile and 1 mmol of thiophenol.b Salicyladehyde (1.5 mmol), malononitrile (3 mmol) and benzenethiol (1.5 mmol).c Isolated yields. | ||||
| 1 | H2O (80 °C) | ≈80 | 200/140 | 55/42 |
| 2 | CH3CN (reflux) | 37.5 | 150/100 | 70/64 |
| 3 | DMF (reflux) | 38 | 160/110 | 65/60 |
| 4 | CHCl3 (reflux) | 4.81 | 260/180 | 31/38 |
| 5 | EtOH (45 °C) | ≈24.5 | 130/98 | 69/60 |
| 6 | EtOH (reflux) | 24.5 | 99/54 | 92/88 |
| Entry | Catalyst | Surface area | Time 5a/6a (min) | Yieldc 5a/6a (%) |
|---|---|---|---|---|
| a 1 mmol of 4-nitrobenzaldehyde, 2.2 mmol of malononitrile and 1 mmol of thiophenol.b Salicyladehyde (1.5 mmol), malononitrile (3 mmol) and benzenethiol (1.5 mmol).c Isolated yields. | ||||
| 1 | SnO bulk | 0.93 m2 g−1 | 190/120 | 63/56 |
| 2 | SnO 28 nm | 35 m2 g−1 | 99/54 | 92/88 |
| 3 | SnO 40 nm | 29.9 m2 g−1 | 106/65 | 89/85 |
| 4 | SnO 82 nm | 16.2 m2 g−1 | 116/73 | 83/81 |
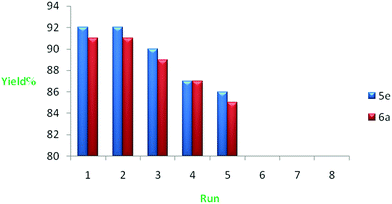
We also investigated recycling of the SnO NPs as catalyst under reflux conditions in ethanol. The results showed that SnO NPs can be reused several times without noticeable loss of catalytic activity (yields 92 to 86%) (Fig. 4). The catalyst could be reused for five times with a minimal loss of activity. Perhaps, activity of SnO NPs is decreased by the number of the regeneration. The morphology and particle size of SnO nanoparticle was investigated by scanning electron microscopy (SEM) before use and after reuse of five times with images shown in Fig. 5. The SEM of SnO nanoparticles before and after the reaction showed identical shape. Interestingly, the morphology of the nanoparticles remained unchanged before and after reaction. We believe that, this is also the possible reason for the extreme stability of the SnO nanoparticles presented herein.
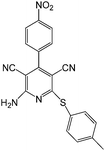
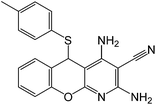
To study the scope of this reaction, we next utilized various aldehydes and thiols in four-component reactions under reflux conditions in ethanol (Table 4).
| Entry | Aldehyde | Thiols | Product of 5a–l and 6a–f | Time (min) | Yielda (%) | M.P °C |
|---|---|---|---|---|---|---|
| a Isolated yields. | ||||||
| 1 |  |
 |
 |
110 | 84 | 229–231 (ref. 26) |
| 2 |  |
 |
 |
125 | 83 | 240–242 (ref. 26) |
| 3 |  |
 |
 |
132 | 79 | 218–220 (ref. 26) |
| 4 | 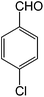 |
 |
 |
141 | 80 | 237–239 (ref. 26) |
| 5 |  |
 |
 |
99 | 92 | 301–303 (ref. 26) |
| 6 | 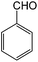 |
 |
 |
103 | 89 | 248–250 (ref. 26) |
| 7 |  |
 |
 |
115 | 81 | 278–280 (ref. 36) |
| 8 |  |
 |
 |
112 | 85 | 206–207 (ref. 37) |
| 9 |  |
 |
 |
100 | 89 | 221–222 (ref. 37) |
| 10 |  |
 |
 |
142 | 79 | 315–317 (ref. 37) |
| 11 |  |
 |
 |
98 | 92 | 289–290 (ref. 37) |
| 12 |  |
 |
 |
104 | 85 | 255–257 (ref. 36) |
| 13 |  |
 |
 |
54 | 88 | 220–222 (ref. 34) |
| 14 |  |
 |
 |
57 | 85 | 223–225 (ref. 34) |
| 15 |  |
 |
 |
62 | 80 | 173–175 |
| 16 |  |
 |
 |
63 | 81 | 200–202 |
| 17 |  |
 |
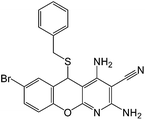 |
56 | 83 | 206–208 (ref. 27) |
| 18 |  |
 |
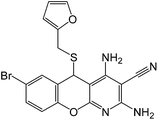 |
57 | 85 | 225–227 (ref. 27) |
As represented in Table 4, these products precipitate from refluxing ethanolic solutions and are isolated by easy filtration.
The proposed mechanism for the preparation of 2-amino-3,5-dicyano-6-sulfanyl pyridines is shown in Scheme 2. Initially, we assumed that the reaction occurs via a Knoevenagel condensation between malononitrile and aldehyde. Then, the subsequent Michael-type addition of the second molecule of malononitrile to the Knoevenagel adduct and thiolate addition to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N of the adduct and cyclization to dihydropyridine which upon aromatization and oxidation (air) under the reaction conditions leads to pyridine. In this mechanism the SnO NPs act as Lewis solid acid and activate the C
N of the adduct and cyclization to dihydropyridine which upon aromatization and oxidation (air) under the reaction conditions leads to pyridine. In this mechanism the SnO NPs act as Lewis solid acid and activate the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group for better reaction with nucleophiles.
O group for better reaction with nucleophiles.
 | ||
| Scheme 2 Possible mechanism of the one-pot reaction for the preparation of 2-amino-3,5-dicyano-6-sulfanyl pyridines. | ||
A possible mechanism for the formation of chromeno[2,3-b]pyridines is shown in Scheme 3. Thus, salicylaldehyde reacts with 1 equiv. of malononitrile to form iminochromene H. This compound undergoes addition with thiophenol to afford phenylsulfanylchromene I. Finally, when I is treated with another equivalent of malononitrile, chromenopyridine P forms in good to excellent yield. Also, the in the present reaction SnO NPs may play as Lewis solid acids. The increased surface area due to small particle size increased reactivity. So, we were encouraged to use SnO NPs in the following optimization of the reaction conditions.
This flexible and nano-catalytic procedure showed good recyclability and provides cleaner condensation reaction in a short reaction time and excellent yields. Nanoparticles exhibit good catalytic activity due to their large surface area and active sites which are mainly responsible for their catalytic activity. These advances have opened the door for the design of fresh nanocatalysts for particular applications in synthetic chemistry.
3. Experimental
3.1. Chemicals and apparatus
All reagents and solvents were purchased from Merck (Germany) and Aldrich and used without further purification. Melting points were determined on Electro thermal 9200.1H NMR and 13C NMR spectra were recorded with a Bruker Avance-400 MHz. NMR spectra were obtained in CDCl3 and DMSO-d6 solution and are reported as parts per million (ppm) downfield from tetramethylsilane as internal standard. The IR spectra were recorded on FT-IR Magna 550 apparatus using with KBr plates. EIMS (70 eV) was performed by Finnigan-MAT-8430 mass spectrometer in m/z. The elemental analyses (C. H. N) of the samples were performed using a LECO CHNS 923 analyser. Surface area was determined by nitrogen adsorption measurement (Micrometrics ASAP-2000). Powder X-ray diffraction (XRD) of SnO nanoparticles was carried out on a Philips diffractometer of X'pert Company with monochromatized Cu Kα radiation (λ = 1.5406 Å). Microscopic morphology was visualized by SEM (LEO 1455VP).3.2. Preparation of SnO nanoparticles
Tin oxide nanoparticles were prepared according to the procedure reported in the literature.35 A 100 ml aqueous solution of 10−2 M is prepared by dissolving 0.225 g of tin(II) chloride (SnCl2·2H2O) in dilute HCl. The solution was continuously stirred and diluted NH4OH was added drop-wise to obtain a precipitate. The solution pH increased to 5. The precipitate was washed several times to remove excess ions. The precipitate dispersed in water kept under irradiation of microwave for 18 min. The sample is characterised by X-ray diffraction (XRD) and scanning electron microscopy (SEM).3.3. General procedure for the preparation of 2-amino-3,5-dicyano-6-sulfanyl pyridines
SnO nanoparticles (6 mol%) were added to a mixture of aldehyde (1 mmol) and malononitrile (2.2 mmol) in 5 mL ethanol and the reaction mixture was stirred for 10 min at 60 °C. Then, the desired thiol (1 mmol) was added to the solution and the solution was refluxed. Progress of the reaction was continuously monitored by thin-layer chromatography. When the reaction was completed, the mixture was cooled to room temperature and centrifuged to separate the catalyst. The solvent was evaporated under vacuum and the solid obtained was recrystallized from ethanol to afford the pure pyridines. The products were characterized by IR, NMR analysis and elemental analysis.3.4. General procedure for the preparation of chromeno[2,3-b]pyridines
To a mixture of a selected salicylaldehyde (1.5 mmol), malononitrile (3 mmol) and a desired thiol (1.5 mmol) in 5 mL of anhydrous ethanol was added SnO NPs (6 mmol%). The resulting mixture was refluxed for 50–60 min and then allowed to cool to room temperature. The formed precipitate was isolated by filtration. The product was dissolved in DMF (3 mL) and the catalyst was filtered. Then, 4 mL water was added to the filtrate which resulted in the crystallization of the product. The resulting crystalline structure was filtered and dried with a vacuum pump. The structures of the products were fully established on the basis of their 1H NMR, 13C NMR and FT-IR spectra.3.5. Spectral data
4. Conclusions
In conclusion, we have developed a flexible, green and highly efficient protocol for the synthesis of chromeno[2,3-b]pyridines and 2-amino-3,5-dicyano-6-sulfanyl pyridines using SnO nanoparticles under reflux conditions in ethanol. These heterocyclic compounds will provide promising candidates for chemical biology and drug discovery. The advantages offered by this method include, easy workup, the employment of a cost-effective catalyst, short reaction times, excellent yields and the use of ethanol as a solvent that is considered to be relatively environmentally benign.Acknowledgements
The authors acknowledge a reviewer who provided helpful insights. The authors are grateful to University of Kashan for supporting this work by Grant no.: 159196/XXI.References
- S. B. Levy, M. N. Alekshun, B. L. Podlogar, K. Ohemeng, A. K. Verma, T. Warchol, B. Bhatia, T. Bowser and M. Grier, Substituted benzoimidazole compounds as transcription factor-modulating compounds useful as anti-infectives, US Patent Appl. 2005124678 A120050609, 2005.
- M. T. Cocco, C. Congiu, V. Lilliu and V. Onnis, Bioorg. Med. Chem., 2007, 15, 1859–1867 CrossRef CAS PubMed.
- T. R. K. Reddy, R. Mutter, W. Heal, K. Guo, V. J. Gillet, S. Pratt and B. Chen, J. Med. Chem., 2006, 49, 607–615 CrossRef CAS PubMed.
- H. Harada, S. Watanuk, T. Takuwa, K. Kawaguchi, T. Okazaki, Y. Hirano and C. Saitoh, PCT Int. Appl. WO Patent 2002006237 A1 20020124, 2002.
- T. Murata, M. Shimada, S. Sakakibara, T. Yoshino, H. Kadono, T. Masuda, M. Shimazaki, T. Shintani, K. Fuchikami, K. Sakai, H. Inbe, K. Takeshita, T. Niki, M. Umeda, K. B. Bacon, K. B. Ziegelbauer and T. B. Lowinger, Bioorg. Med. Chem. Lett., 2003, 13, 913 CrossRef CAS.
- J. Deng, T. Sanchez, L. Q. Al-Mawsawi, R. Dayam, R. A. Yunes, A. Garofalo, M. B. Bolger and N. Neamati, Bioorg. Med. Chem., 2007, 15, 4985 CrossRef CAS PubMed.
- H. Chen, W. Zhang, R. Tam and A. K. Raney, PCT Int. Appl. WO2005058315 A1 20050630, 2005.
- M. W. Beukers, L. C. W. Chang, J. K. F. D. Künzel, T. Mulder-Krieger, R. F. Spanjersberg, J. Brussee and A. P. Ijzerman, J. Med. Chem., 2004, 47, 3707–3709 CrossRef CAS PubMed.
- L. C. W. Chang, J. K. Künzel, T. Mulder-Krieger, R. F. Spanjersberg, S. F. Roerink, G. Van Den Hout, M. W. Beukers, J. Brussee and A. P. Ijzerman, J. Med. Chem., 2004, 48, 2045–2053 CrossRef PubMed.
- B. B. Fredholm, A. P. Ijzerman, K. A. Jacobson and K.-N. Klotz, Pharmacol. Rev., 2001, 53, 527–552 CAS.
- S. K. Srivastava, R. P. Tripathi and R. Ramachandran, J. Biol. Chem., 2005, 280, 30273–30281 CrossRef CAS PubMed.
- G. Kolokythas, N. Pouli, P. Marakos, H. Pratsinis and D. Kletsas, Eur. J. Med. Chem., 2006, 41, 71–79 CrossRef CAS PubMed.
- Y. Maruyama, K. Goto and M. Terasawa, Ger. Offen. DE Patent 3010751 19810806, 1981.
- M. A. Azuine, H. Tokuda, J. Takayasu, F. Enjyo, T. Mukainaka, T. Konoshima, H. Nishino and G. Kapadia, J. Pharmacol. Res., 2004, 49, 161–169 CrossRef CAS PubMed.
- S. Toshiro and W. Noriko, Eur. Pat. Appl. EP647445 A1 19950412, 1995.
- Y. Ito, H. Kato, S. Yasuda, N. Kato, N. Iwasaki and H. Nishino, M. Takeshita, Jpn. Kokai Tokkyo Koho JP 06107664 A2 19940419, 1994.
- K. Ukawa, T. Ishiguro, H. Kuriki and A. Nohara, Chem. Pharm. Bull., 1985, 33, 4432–4437 CrossRef CAS.
- D. R. Anderson, S. Hegde, E. Reinhard, L. Gomez, W. F. Vernier, L. Lee, S. Liu, A. Sambandam, P. A. Snider and L. Masih, Bioorg. Med. Chem. Lett., 2005, 15, 1587–1590 CrossRef CAS PubMed.
- J. A. Bristol, E. H. Gold, I. Gross, R. G. Lovey and J. F. Long, J. Med. Chem., 1981, 24, 1010–1013 CrossRef CAS.
- J.-P. Wan and Y. Liu, RSC Adv., 2012, 2, 9763–9777 RSC.
- M. S. Singh and S. Chowdhury, RSC Adv., 2012, 2, 4547–4592 RSC.
- F. Yu, R. Huang, H. Ni, J. Fan, S. Yan and J. Lin, Green Chem., 2013, 15, 453–462 RSC.
- A. R. Kiasat and J. Davarpanah, J. Mol. Catal. A: Chem., 2013, 373, 46–54 CrossRef CAS PubMed.
- H. R. Shaterian, M. Ghashang and M. Feyzi, Appl. Catal., A, 2008, 345, 128–133 CrossRef CAS PubMed.
- M. Sridhar, B. C. Ramanaiah, C. Narsaiah, B. Mahesh, M. Kumaraswamy, K. K. R. Mallu, V. M. Ankathi and P. S. Rao, Tetrahedron Lett., 2009, 50, 3897–3900 CrossRef CAS PubMed.
- J. Safaei-Ghomi and M. A. Ghasemzadeh, J. Sulfur Chem., 2013, 34, 233–241 CrossRef CAS.
- J. Safaei-Ghomi, M. Kiani, A. Ziarati and H. Shahbazi-Alavi, J. Sulfur Chem., 2014, 35, 450–457 CrossRef CAS.
- K. Tanabe, Solid acids and bases, Academic Press, New York, 1970 Search PubMed.
- M. Kidwai, A. Jain and S. Bhardwaj, Mol. Diversity, 2012, 16, 121–128 CrossRef CAS PubMed.
- S. Banerjee, A. Horn, H. Khatri and G. Sereda, Tetrahedron Lett., 2011, 52, 1878–1881 CrossRef CAS PubMed.
- A. Maleki, Tetrahedron, 2012, 68, 7827–7833 CrossRef CAS PubMed.
- A. I. Ahmed, S. A. El-Hakam, M. A. A. Elghany and W. S. A. El-Yazeed, Appl. Catal., A, 2011, 407, 40–48 CrossRef CAS PubMed.
- N. M. Evdokimov, A. S. Kireev, A. A. Yakovenko, M. Y. Antipin, I. V. Magedov and A. Kornienko, Tetrahedron Lett., 2006, 47, 9309–9312 CrossRef CAS PubMed.
- S. Mishra and R. Ghosh, Synth. Commun., 2012, 42, 2229–2244 CrossRef CAS.
- M. H. M. Ara, P. Boroojerdian, Z. Javadi, S. Zahedi and M. Morshadian, Micro Nano Lett., 2011, 6, 249–252 CAS.
- R. Mamgain, R. Singh and D. S. Rawat, J. Heterocycl. Chem., 2009, 46, 69–73 CrossRef CAS.
- P. V. Shinde, V. B. Labade, B. B. Shingate and M. S. Shingare, J. Mol. Catal. A: Chem., 2011, 336, 100–105 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra04769a |
| This journal is © The Royal Society of Chemistry 2014 |