Generation of oxygen vacancies in visible light activated one-dimensional iodine TiO2 photocatalysts†
Abstract
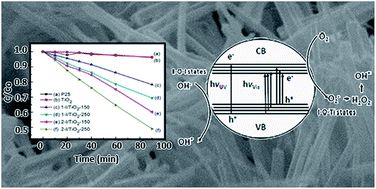
A facile and efficient way of generating oxygen vacancies in visible light activated one-dimensional iodine doped TiO2 photocatalysts was first reported in this work. A two-step hydrothermal synthesis was used to synthesize TiO2 nanomaterials modified by iodic acid (HIO3) as a dopant. Detailed analysis was conducted to illustrate the intrinsic doping/reaction mechanisms of iodic acid in the modification of the TiO2 matrix. The phase and structure evolution were deduced from X-ray diffraction (XRD), Raman, and scanning electron microscopy (SEM). X-ray photoelectron spectroscopy (XPS) was conducted to analyze the generation of oxygen vacancies and the formation of I–O–Ti bonds in the TiO2 lattice. Multi-valences of iodine, due to the reduction of iodic acid, facilitated the generation of oxygen vacancies and 3d state Ti3+ species in the TiO2 lattice. The visible light absorption and enhanced photocatalytic activity of the TiO2 nanomaterials were attributed to existing oxygen vacancies, iodine multi-valences in I–O–Ti bonds, and 3d state Ti3+ sites in the TiO2 lattice. The photocatalytic degradation efficiency under visible light (λ > 400 nm) followed a pseudo first-order kinetic model. Rutile nanowires using a two-step synthesis method produced the highest methylene blue (10 mg L−1) degradation rate constant, Kap, of 7.92 × 10−3 min−1 compared to other synthesized nanomaterials. The Kap value obtained was an order of magnitude greater than commercial P25 (3.87 × 10−4 min−1) and pristine TiO2 nanowires (4.18 × 10−4 min−1). The iodine doped TiO2 photocatalysts can be used in TiO2/light irradiation advanced oxidation processes (AOPs) in water treatment using sunlight or a visible light source, rather than an ultraviolet irradiation source.

 Please wait while we load your content...
Please wait while we load your content...