Controllable synthesis of ultrasmall CuInSe2 quantum dots for photovoltaic application†
Abstract
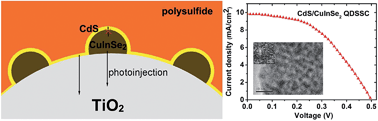
Here we report the first synthesis of ultrasmall CuInSe2 quantum dots (QDs) with diameters below the exciton Bohr radius 10.6 nm by a solvothermal method. The synthesis is conducted in oleylamine without any organometallic precursors. The quantum confinement effect has been identified in the optical absorption spectra. Through pre-loading CuInSe2 QDs on TiO2 film, a good electron transfer dynamics could be observed on the CdS/CuInSe2/TiO2 film. Under one sun of simulated irradiation, the resultant quantum dot sensitized solar cell based on CdS/CuInSe2 exhibited a power conversion efficiency of about 2.27%, which was 55% higher than that of the single CdS sensitized solar cell. It indicates that CuInSe2 QDs have great potential in photovoltaic applications.

 Please wait while we load your content...
Please wait while we load your content...