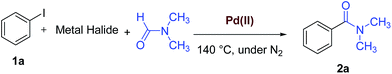WCl6/DMF as a new reagent system for the phosphine-free Pd(0)-catalyzed aminocarbonylation of aryl halides†
Nasser Iranpoor*,
Habib Firouzabadi*,
Zeinab Tavangar Rizi and
Soodabeh Erfan
Chemistry Department, College of Sciences, Shiraz University, Shiraz, Iran. E-mail: iranpoor@susc.ac.ir; firouzabadi@susc.ac.ir; Fax: +987116460788; Tel: +987116460724
First published on 4th September 2014
Abstract
WCl6 in dimethyl formamide (DMF) is introduced as a new reagent system for aminocarbonylation of aryl halides in the presence of PdCl2 as pre-catalyst without any phosphorous ligand. Aryl iodides, bromides as well as chlorides were efficiently converted to their corresponding N,N-dimethyl amides in good to high yields. In this protocol, WCl6/DMF is responsible for the generation of both Pd(0) catalyst as well as the formation of a Vilsmeier imminium type intermediate.
Introduction
The carbonylation reaction is among the effective transformations for the synthesis of esters, amides, and heterocyclic compounds. Among the carbonyl compounds, amides are one of the important functional groups, due to their wide ranging biological activities as antibacterial and antifungal agents.1 The synthesis of amides from aryl halides in the presence of transition metal catalysts is an important reaction in organic synthesis. Regarding this synthesis, Heck has reported the first preparation of aryl and alkenyl amides from their corresponding halides in the presence of palladium catalyst, CO gas, and primary amines.2 After this report, some other metal catalyzed methods were developed for aminocarbonylation of aryl halides in the presence of CO gas.3,4 In order to remove the handling problem of carbon monoxide, the use of Mo(CO)6,5 Cr(CO)6,5 W(CO)6,5 carbamoylstannanes6 and carbamoylsilanes,7 as sources for the in situ generation of carbon monoxide was considered as a practical method. However, the use of metal carbonyls, still have some handling problem due to the generation of CO gas, and in the other hand carbamoylstannane and carbamoylsilanes have thermal instability and are not commercially available.The use of DMF as source of carbonyl group in transition metal catalyzed aminocarbonylation reaction of aryl halides with amines can be considered as an alternative reagent system for amide formation.8–10 This reaction in strongly basic condition has been reported both under thermal8 and microwave irradiation.9 Hallberg et al. reported the Pd-catalyzed aminocarbonylation using DMF as source of CO and an amine.9 However, in this reaction, imidazole was added as an additive and the reaction temperature was very high (180–190 °C). The use of excess POCl3 in DMF was also reported as an example of CO-free aminocarbonylation of aryl and alkenyl halides.10 Later on, this reagent system was used for aminocarbonylation reaction of aryl halides in the presence of Pd/C catalyst.11 The main disadvantage of the mentioned methods is their limited application to only aryl iodides. Most recently, the use of Pd(OAc)2/xantphos as catalyst for CO-free aminocarbonylation of aryl halides in the presence of POCl3 has been reported for preparation of different formamides.12
Recently, we have reported the aminocarbonylation of aryl halides using the in situ generated Mo(CO)4NBD13 and also the use of POCl3/DMF in the presence of nano-palladium catalyst Pd(0)/SDPP (SDPP = silicadiphenylphosphinite) for amide formation.14 In continuation of our recent studies on the amidation of aryl halides, herein, we introduce a new palladium-catalyzed aminocarbonylation of aryl halides using WCl6/DMF as a new combinatorial carbonylation reagent system.
Results and discussion
In order to obtain appropriate conditions for the aminocarbonylation of aryl halides using DMF as CO source, we decided to use different metal halides such as WCl6, MoCl5, ZrOCl2, ZrCl4, TiCl4, and FeCl3 in the presence of Pd(II) as pre-catalyst. The effect of different factors on aminocarbonylation of iodobenzene are demonstrated in Table 1. As shown in this table, among the studied metal halides, WCl6 is the most efficient one and gave quantitative conversion within 5 h. The use of Pd(OAc)2 instead of PdCl2 as a pre-catalyst was also examined and the obtained result showed that the reaction time is nearly the same as using PdCl2 (Table 1, entry 4). Since, PdCl2 is cheaper than Pd(OAc)2, it was selected as the Pd precursor. The influence of parameters such as temperature and catalyst loading was also examined on the model reaction.| Entry | Metal halide | Pd(II) (mol%) | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: iodobenzene (0.5 mmol), metal halide (1.0 mmol), DMF (5.0 mL).b Conversion yield was based on GC analysis.c Biphenyl was obtained as the product.d Pd(OAc)2 was used instead of PdCl2.e The reaction was performed at 120 °C.f One equimolar of WCl6 was used.g 2.0 mg of PPh3 was used. | ||||
| 1 | WCl6 | None | 24 | 0 |
| 2 | None | 2.5 | 24 | 30c |
| 3 | WCl6 | 2.5 | 5 | 100 |
| 4 | WCl6 | 2.5 | 4.5 | 100d |
| 5 | MoCl5 | 2.5 | 8.5 | 100 |
| 6 | ZrOCl2 | 3.5 | 12 | 100 |
| 7 | ZrCl4 | 5 | 24 | 70 |
| 8 | FeCl3 | 5 | 24 | None |
| 9 | TiCl4 | 5 | 24 | None |
| 10 | WCl6 | 2.5 | 18 | 45e |
| 11 | WCl6 | 2.5 | 12 | 90f |
| 12 | WCl6 | 2.5 | 12 | 100g |
Running the reaction with optimized 2.5 mol% of PdCl2 in the presence of WCl6 as the reagent at 120 °C showed that the yield of the desired product was too low after 18 h (45% GC conversion) (Table 1, entry 10).
As a controlled reaction, we also checked the aminocarbonylation reaction in the absence of Pd catalyst and once in the absence of metal halide. In the absence of Pd catalyst, the reaction did not proceed even after 24 h (Table 1, entry 1). In the absence of WCl6, most of the starting material was remained intact after 24 h and biphenyl wao obtained as by-product in 30% yield (Table 1, entry 2). From these experiments, it was concluded that the presence of both Pd catalyst and WCl6 are essential to produce the corresponding product. When the amounts of WCl6 was reduced from two equivalents to one, the reaction time was increased from 5 to 12 h (Table 1, entry 11). In order to see the effect of phosphorous ligand on the process of the reaction, the model reaction was conducted in the presence of PPh3 (Table 1, entry 12). In comparison with the ligand-free reaction (Table 1, entry 3), the presence of PPh3 not only didn't improve the progress of the reaction, but also increased the reaction time. The elongation of reaction time could be possibly due to the complexation of PPh3 with WCl6.
Since it was the first time that WCl6/DMF is used for this reaction, we decided to find out its scope and applicability for aminocarbonylation of aryl halides. We therefore, studied the possibility of performing this new aminocarbonylation reaction in the presence of WCl6 as the most efficient metal halide and apply this ligand-free reaction to other aryl halides. Under our optimized reaction conditions (0.5 mmol of aryl halide, 1.0 mmol of WCl6 5.0 mL of DMF, 4.4 mg (2.5 mol%) of PdCl2 under nitrogen at 140 °C), the desired products were obtained in moderate to excellent yields for a wide array of aryl halides (Scheme 1).
As shown in Scheme 1, different aryl halides can be converted to their corresponding amid derivatives under our optimized conditions in the presence of WCl6/DMF reagent system. Aryl iodides reacted faster than bromides and chlorides counterparts. The reaction conditions are more effective for aryl halides containing electron-withdrawing groups so that electron-poor substrates reacts faster than electron-rich ones.
Steric hindrance in some substrates such as 1-iodonaphthalene, 1-iodo-2-methylbenzene and 1-iodo-2-methyl-4-nitrobenzene caused a decrease in the yield of the desired product (Scheme 1, compounds 2e–g). This catalytic system was also efficient for the electron-deficient aryl bromides and aryl chlorides (Scheme 1, compounds 2h–i).
In order to see if the reaction can be applied to other formamides, we used N,N-diethylformamide (DEF) instead of DMF for aminocarbonylation of some aryl iodides under optimized conditions. Unfortunately, we couldn't obtain any product from this reaction. Interestingly, the presence of PPh3 as ligand is required for occurrence of the reaction. Using DEF, iodobenzene and 4-methoxy iodobenzene reacted efficiently to give their corresponding N,N-diethylamides in high yields under optimized conditions, but in the presence of PPh3 ligand (Scheme 1, compounds 2j, k).
In order to study the pathway of Pd(II) reduction under phosphine-free condition, we studied the following experiments. First, we added PdCl2 to the solution of WCl6 in DMF and heated it at 140 °C for 1 h or 80 °C for 3 h. Then we studied the UV-spectrum of both solutions. Disappearance of the band around 400 nm was indicative of the absence of Pd(II) under these conditions. In the other experiment in order to see the effect of DMF, a combination of PdCl2 and DMF was stirred at 140 °C for 3 h in the absence of WCl6. The UV-spectrum of this mixture showed that no reduction of Pd(II) to Pd(0) has been occurred (Fig. 1a).
 | ||
| Fig. 1 (a) UV-spectrum of PdCl2 in the presence of DMF and WCl6. (b) UV-spectrum of PdCl2 in the presence of DMF and MoCl5. (c) UV-spectrum of PdCl2 in the presence of DMF and ZrOCl2. | ||
In order to have a comparison with other metal halides, we also performed a similar experiment sing MoCl5 in DMF at 140 °C. The UV spectrum of the mixture showed that Pd(II) was completely converted to Pd(0) after 2 h which is slightly slower than using WCl6 (Fig. 1b). When we studied ZrOCl2 for the same purpose, it was observed that after 2 h, Pd(II) was still present in the media, which shows that the reduction of Pd(II) to Pd(0) is much slower in the case of ZrOCl2 (Fig. 1c). This study clearly shows that the reduction of Pd(II) to Pd(0) occurs more efficiently in WCl6/DMF.
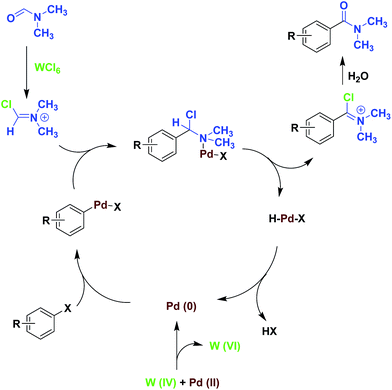
According to the reported results in the literature, DMF has been shown to act as reducing agent for the conversion of Ag(I) to Ag(0).15 On this basis, it can be suggested that DMF can act as a reducing agent for the conversion of W(VI) to W(IV) as shown in Fig. 2. In the following, the produced W(IV) acts as a reducing agent for the reduction of Pd(II) to Pd(0). Detection of dimethyl amine by gc analysis in the reaction mixture is an evidence for the proposed mechanism.
In order to show that the formation of dimethyl amine occurs through the reduction processes and is not a thermic decomposition product of DMF, the mixture of WCl6 in DMF was also heated at 80 °C in a sealed tube and its gc analysis was compared with a blank solution of DMF under similar condition. The formation of dimethyl amine in the mixture of WCl6/DMF and its absence in the blank solution confirms the proposed pathway for the generation of dimethyl amine.
Since formation of Pd(0) from Pd(II) was not observed in DEF, it can be concluded that DEF can not act as a reducing agent for the conversion of Pd(II) to Pd(0), thus we needed to add PPh3 as a phosphine ligand to the reaction mixture for the reduction of Pd(II) to generate the Pd(0) catalyst.
Although the mechanism for this reaction is not clear at this time, we propose a plausible reaction pathway according to our findings and also the litrature as shown in Scheme 2.10,15 In the first step, reduction of WCl6 in DMF is occurred and W(IV) is possibly formed. Then this compound can reduce Pd(II) to Pd(0). The Vilsmeier imminium salt reagent [Me2N+ = CHCl] is also produced from the reaction of DMF and WCl6. The Intermediate I which is produced from the oxidative addition of aryl halide to Pd(0) reacts with the Vilsmeier imminium salt. Then the reaction proceeds via the Heck-type addition of aryl halides to the imminium species as shown in Scheme 2.
Since formation of the Vilsmeier imminium salt reagent [Me2N+ = CHCl] is the required step in this catalytic cycle, the use of at least stoichiometric amount of WCl6 is required to generate this intermediate through the reaction with DMF.
In order to show the formation of Vilsmeier imminium reagent proposed in the mechanism, in an experiment, indole (1.0 mmol) was reacted with WCl6 (1.0 mmol) in DMF (2 mL) at 80 °C. After 2 h, indol 3-carbaldehyde was obtained in 75% isolated yield. This experiment strongly confirms the generation of Vilsmeier imminium reagent under our optimized conditions (Scheme 3).
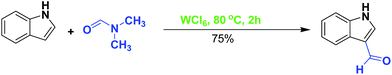 | ||
| Scheme 3 Synthesis of indole-3-carbaldehyde representing the formation of Vilsmeier imminium reagent from DMF/WCl6 system. | ||
Conclusions
In this study, we have introduced a new method for aminocarbonylation of aryl halides in the presence of WCl6 as an efficient metal halide in the presence of catalytic amounts of PdCl2 as precatalyst in the absence of any phosphine ligand. For this transformation, N,N-dimethylformamide (DMF) has been used as the source of carbonyl group. Among the studied metal halides, WCl6 showed to be the most efficient one. A possible reason for the more efficiency of WCl6 was shown to be due to the easier reduction of Pd(II) to Pd(0) in WCl6/DMF compared with those reagent systems using MoCl5, ZrOCl2 or ZrCl4 in DMF. The reaction of aryl iodides in DEF can also occure to give the corresponding N,N-diethyl amides but in the presence of PPh3 as a reducing ligand.Experimental section
The progress of reactions was followed by TLC on silica gel SILG/UV 254 plates or GLC analysis on a Shimadzu model GC-10A instrument. IR spectra were run on a Shimadzu FTIR-8300 spectrophotometer. The 1H NMR and 13C NMR spectra were recorded on a Bruker-Avance DPX 250 FT-NMR spectrometer.General procedure for aminocarbonylation of aryl halides with WCl6 in the presence of PdCl2 as catalyst in DMF
WCl6 (1.0 mmol, 0.415 g) and dry DMF (5.0 mL) were added to a flask containing a magnetic stirring bar. The resulting mixture was stirred for 15 min at room temperature under nitrogen. Then aryl halide (0.5 mmol), and PdCl2 (2.5 mol%, 4.4 mg) were added to the reaction mixture. After 15 min, the mixture was heated at 140 °C for the appropriate time. After the consumption of the starting material (monitored by TLC or GC analysis), the reaction mixture was cooled down to room temperature. The resulting mixture was added to a saturated aqueous solution of NaHCO3 (25 mL). The organic layer was extracted with ethyl acetate (25 mL × 4) and then the solvent was evaporated to obtain the desired product. Further purification was performed by silica gel column chromatography [60 Merck (230–240 mesh)] using n-hexane/ethyl acetate (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent to give the pure product in moderate to excellent yields.
1) as the eluent to give the pure product in moderate to excellent yields.
General procedure for aminocarbonylation of aryl halides with WCl6 in the presence of PdCl2/PPh3 as catalyst in N,N-diethylformamide
WCl6 (1.0 mmol, 0.396 g) was dissolved in N,N-diethylformamide (5.0 mL). Then, aryl halide (0.5 mmol), PdCl2 (2.5 mol%, 4.4 mg) and PPh3 (2.0 mg) were added to the vessel containing the mixture of DEF and WCl6. After 15 min, the mixture was stirred for the specified time at 140 °C under nitrogen (Table 1). After consumption of the starting material (TLC or GC), the reaction mixture was cooled down to room temperature. Saturated solution of NaHCO3 (25 mL) was added to the reaction mixture and was then extracted with ethyl acetate (25 × 4 mL). Evaporation of the solvent gave the desired pure amide. Further purification, if was necessary, was performed by silica gel column chromatography using n-hexane/EtOAc as the eluent to give the pure product in high to excellent yield.Acknowledgements
The authors would like to acknowledge the support of this work by Shiraz University Research Council and the grant from Iran National Elite Foundation. Technical assistance of Dr S. Motevalli is also acknowledged.Notes and references
- (a) K. J. Pradhan, P. S. Variyar and J. R. Bandekar, Lebensm.-Wiss. Technol., 1999, 32, 121–123 CrossRef CAS; (b) E. Aki-Sener, K. K. Bingol, I. Oren, O. Temiz-Arpaci, I. Yalcin and N. Altanlar, Farmaco, 2000, 55, 469–476 CrossRef; (c) I. Kobayashi, H. Muraoka, M. Hasegawa, T. Saika, M. Nishida, M. Kawamura and R. J. Ando, J. Antimicrob. Chemother., 2002, 50, 129–132 CrossRef CAS PubMed; (d) J. M. Humphrey and A. R. Chamberlin, Chem. Rev., 1997, 97, 2243–2266 CrossRef CAS PubMed.
- A. Schoenberg and R. F. Heck, J. Org. Chem., 1974, 39, 3327–3331 CrossRef CAS.
- Z. S. Qureshi, S. A. Revankar, M. V. Khedkar and B. M. Bhanage, Catal. Today, 2012, 198, 148–153 CrossRef CAS PubMed.
- (a) M. Beller, B. Cornils, C. D. Frohning and C. W. Kohlpaintner, J. Mol. Catal. A: Chem., 1995, 104, 17–85 CrossRef CAS; (b) A. Yamamoto, Y. Kayaki, K. Nagayama and I. Shimizu, Synlett, 2000, 925–937 CAS.
- (a) N. F. K. Kaiser, A. Hallberg and M. Larhed, J. Comb. Chem., 2002, 4, 109–111 CrossRef CAS; (b) J. Wannbeg and M. Larhed, J. Org. Chem., 2003, 68, 5750–5753 CrossRef PubMed.
- C. M. Lindsay and D. A. Widdowson, J. Chem. Soc., Perkin Trans. 1, 1988, 569–573 RSC.
- (a) R. F. Cunico and B. C. Maity, Org. Lett., 2002, 4, 4357–4359 CrossRef CAS PubMed; (b) R. F. Cunico and B. C. Maity, Org. Lett., 2003, 5, 4947–4949 CrossRef CAS PubMed.
- J. Ju, M. Jeong, J. Moon, H. M. Jung and S. Lee, Org. Lett., 2007, 9, 4615–4618 CrossRef CAS PubMed.
- Y. Wan, M. Alterman, M. Larhed and A. Hallberg, J. Org. Chem., 2002, 67, 6232–6235 CrossRef CAS PubMed.
- K. Hosoi, K. Nozaki and T. Hiyama, Org. Lett., 2002, 4, 2849–2851 CrossRef CAS PubMed.
- P. J. Tambade, Y. P. Patil, M. J. Bhanushali and B. M. Bhanage, Tetrahedron Lett., 2008, 49, 2221–2224 CrossRef CAS PubMed.
- D. N. Sawant, Y. S. Wagh, K. D. Bhatte and B. M. Bhanage, J. Org. Chem., 2011, 76, 5489–5494 CrossRef CAS PubMed.
- N. Iranpoor, H. Firouzabadi, S. Motevalli and M. Talebi, Tetrahedron, 2013, 69, 418–426 CrossRef CAS PubMed.
- N. Iranpoor, H. Firouzabadi and S. Motevalli, J. Mol. Catal. A: Chem., 2012, 355, 69–74 CrossRef CAS PubMed.
- I. Pastoriza-Santos and L. M. Liz-Marzán, Pure Appl. Chem., 2000, 72, 83–90 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Copy of 1H NMR and 13C NMR spectra of compounds. See DOI: 10.1039/c4ra04673k |
| This journal is © The Royal Society of Chemistry 2014 |