Study on the interaction between emodin and ethyl violet by resonance Rayleigh scattering technique†
Abstract
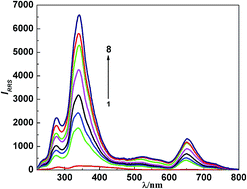
A novel resonance Rayleigh scattering method was developed for the determination of emodin (EMO). In pH 7.0 Britton–Robinson (BR) buffer medium, the scattering signal of ethyl violet was remarkably enhanced after adding trace amount of EMO and forming a 1 : 1 ion-association complex, which not only resulted in the change of absorption spectra, but also led to a significant enhancement of resonance Rayleigh scattering (RRS), frequency doubling scattering (FDS) and second order scattering (SOS). The maximum RRS, SOS and FDS wavelengths of the ion-association complex were located at 340 nm, 528 nm and 341 nm, respectively. The linear ranges and detection limits for RRS, SOS and FDS were 0.1–4.2 μg mL−1, 0.4–4.8 μg mL−1, 0.4–4.8 μg mL−1 and 3.8 ng mL−1, 14.2 ng mL−1, 17.7 ng mL−1, respectively. In this work, the optimum conditions, the influencing factors and the effects of coexisting substances on the reaction were investigated. The method can be applied to the determination of EMO in serum and urine sample and the results were satisfactory. Moreover, the reaction mechanism and reasons of the enhancement of resonance light scattering were discussed.

 Please wait while we load your content...
Please wait while we load your content...