Synthesis of mono-dispersed mesoporous SBA-1 nanoparticles with tunable pore size and their application in lysozyme immobilization†
Abstract
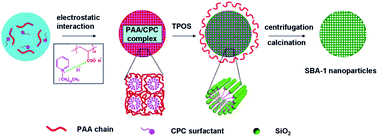
A simple synthesis of mesoporous silica nanoparticles with cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n pore structure (SBA-1) and controlled pore size is reported. The synthesis of mesoporous silica nanoparticles is achieved by employing organic mesomorphous complexes of polyelectrolyte (poly(acrylic acid)) and cationic surfactant (hexadecyl pyridinium chloride) as template, and tetrapropoxysilane as silica source. It is found that the pore size of the synthesized mesoporous silica nanoparticles can be tuned by the incorporation of 1,3,5-trimethylbenzene. By adjusting the amount of 1,3,5-trimethylbenzene in the synthesis, a series of silica nanoparticles with controlled pore size ranging from 2.5 nm to 5.3 nm is obtained. The adsorption properties of the mesoporous silica nanoparticles with different pore size against lysozyme are compared. The results show that mesoporous silica nanoparticles with proper pore size and well ordered pore structure benefit the adsorption and exhibit a low leaching rate.
n pore structure (SBA-1) and controlled pore size is reported. The synthesis of mesoporous silica nanoparticles is achieved by employing organic mesomorphous complexes of polyelectrolyte (poly(acrylic acid)) and cationic surfactant (hexadecyl pyridinium chloride) as template, and tetrapropoxysilane as silica source. It is found that the pore size of the synthesized mesoporous silica nanoparticles can be tuned by the incorporation of 1,3,5-trimethylbenzene. By adjusting the amount of 1,3,5-trimethylbenzene in the synthesis, a series of silica nanoparticles with controlled pore size ranging from 2.5 nm to 5.3 nm is obtained. The adsorption properties of the mesoporous silica nanoparticles with different pore size against lysozyme are compared. The results show that mesoporous silica nanoparticles with proper pore size and well ordered pore structure benefit the adsorption and exhibit a low leaching rate.

 Please wait while we load your content...
Please wait while we load your content...