Multifarious zinc coordination polymers based on biphenyl-3,3′,5,5′-tetracarboxylate and different flexibility of N-donor ligands†
Qipeng Li*ab and
Jinjie Qianab
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: qpli@fjirsm.ac.cn; Fax: +86-591-83709847
bUniversity of Chinese Academy of Sciences, Beijing 100039, P. R. China
First published on 8th July 2014
Abstract
Based on different flexibility of N-donor and biphenyl-3,3′,5,5′-tetracarboxylate ligands we successfully construct four novel Zn(II) coordination polymers, formulated as {[Zn2(bptc)(4,4′-bpy) (H2O)2]·2H2O}n (1), {[Zn(H2bptc)(bpe)]·2H2O}n (2), {[Zn2(bptc)(bpmp)2]·2H2O}n (3) and {[Zn2(bptc)(biim-4)2]·H2O}n (4) [H4bptc = biphenyl-3,3′,5,5′-tetracarboxylic acid, 4,4′-bpy = 4,4′-bipyridine, bpe = 1,2-bis(4-pyridyl)ethane, bpmp = N,N′-bis-(4-pyridyl-methyl) piperazine and biim-4 = 1,1′-(1,4-butanediyl)bis(imidazole)]. Single-crystal X-ray diffraction studies reveal that these four coordination polymers present various topological supramolecular architectures originated from auxiliary N-donor ligands with different flexibility. Complex 1 displays a 3D supramolecular polymer based on the rigid 4,4′-bpy ligands. Complex 2 shows a twofold 2D → 3D framework by parallel polycatenation of undulating 2D sheets based on the semi-rigid bpe ligands. Complex 3 exhibits an unusual 3D self-penetrating coordination framework constructed from the semi-rigid bpmp ligands. Complex 4 is a unique threefold 3D interpenetrated framework based on the flexible biim-4 ligands. In addition, their solid-state luminescent properties also have been systematically investigated.
Introduction
Various metal centers and multifunctional organic ligands have been used for the design and construction of metal–organic frameworks (MOFs) and coordination polymers (CPs) due to their potential application as functional materials in the fields of gas absorption and separation, ion-exchange, catalysis, luminescence and magnetism.1–5 To date, a large number of MOFs and CPs with fascinating structure and diverse topologies have been rationally assembled via transition or rare-earth metals of high coordination numbers and organic ligands with multidentate carboxylate functionalities.6–9 Over the past few decades, much efforts have been devoted for investigating the effects on the introduction of various symmetry-related aromatic carboxylate ligands with different sizes on MOFs and CPs.10 Such as, 1,3,5-benzenetricarboxylic acid (H3BTC) and 1,3,5-tris(4-carboxyphenyl)benzene (H3BTB) as O-donor ligands, which play a crucial role in self-assembly of MOFs and CPs with diverse multidimensional architectures.11,12 Simultaneously, the effects of neutral N-donor ligands with different flexibility on process of directing self-assembly have also attracted more and more attentions.13 In addition, the d10 metal ions are often used to construct beautiful architectures because their MOFs or CPs exhibit outstanding photoluminescence properties and so forth.14By utilizing a mixed ligand system of H4bptc and the rigid, semi-rigid and flexible N-donor ligands (Scheme 1) to construct Zn(II)-based CPs with desirable topology and luminescence properties, we successfully work out four examples Zn(II)-based CPs, namely {[Zn2(bptc)(4,4′-bpy) (H2O)2]·2H2O}n (1), {[Zn(H2bptc)(bpe)]·2H2O}n (2), {[Zn2(bptc)(bpmp)2]·2H2O}n (3) and {[Zn2(bptc)(biim-4)2]·H2O}n (4). Although few examples of Zn(II)-based CPs based on H4bptc and N-donor ligands have been reported, but the systemic research on the N-donor ligands with different flexibility have never been reported so far.15 Herein, their detailed syntheses, crystal structures and luminescent properties were discussed.
Experimental section
Materials and methods
Reactions were carried out in 23 ml glass vials under autogenous pressure. All the starting materials and solvents were available commercially sources and used as purchased without further purification. The power X-ray diffraction (PXRD) patterns were collected by a Rigaku DMAX2500 X-ray Diffractometer using Cu Kα radiation (λ = 0.154 nm). Fluorescent analyses of the complexes were performed on Fluorolog-3. Thermogravimetric analyses (TGA) were recorded on a NETZSCH STA 449C unit at a heating rate of 10 °C min−1 under flowing nitrogen atmosphere. Elemental analyses for C, H and N were carried out on a German Elementary Vario EL III instrument. The FT-IR spectra were performed on a Nicolet Magna 750 FT-IR spectrometer using KBr pellets in the range of 4000–400 cm−1.Synthesis of {[Zn2(bptc)(4,4′-bpy)(H2O)2]·2H2O}n (1)
A mixture of Zn(NO3)2·6H2O (0.10 mmol, 30 mg), H4bptc (0.10 mmol, 33 mg) and 4,4′-bpy (0.10 mmol, 16 mg) in N,N′-dimethylformamide (DMF) (5 ml) and H2O (5 ml) with an additional 0.1 ml HNO3 (w.t. 65%) was placed in a 23 ml vial, which was heated at 85 °C for 2 days and cooled to room temperature. After washed by ethanol, the colorless crystals were obtained in ca. 60% yield based on Zn(NO3)2·6H2O. Anal. calcd for C26H22N2O12Zn2 (Mr = 685.20): C, 45.57%; H, 3.24%; N, 4.06%. Found: C, 45.58%; H, 3.21%; N, 4.22%. Selected IR data (KBr pellet, cm−1): 3088 (s) 1860 (w) 1615 (s) 1568 (s) 1412 (s) 1354 (s) 1227 (m) 1071 (m) 820 (m) 772 (s) 734 (s) 663 (m) 645 (m) 570 (m) 460 (w).Synthesis of {[Zn(H2bptc)(bpe)]·2H2O}n (2)
The preparation of 2 was similar to 1 except that 0.10 mmol of 4,4′-bpy was replaced by 0.10 mmol bpe. The colorless crystals were obtained in ca. 75% yield based on Zn(NO3)2·6H2O. Anal. calcd for C28H22N2O10Zn (Mr = 611.85): C, 54.96%; H, 3.62%; N, 4.58%. Found: C, 54.70%; H, 3.90%; N, 4.63%. Selected IR data (KBr pellet, cm−1): 3508 (b) 1713 (s) 1636 (s) 1614 (s) 1553 (m) 1430 (m) 1348 (m) 1309 (s) 1229 (s) 1157 (w) 1091 (w) 1029 (m) 837 (w) 761 (m) 703 (w) 656 (w) 551 (w).Synthesis of {[Zn2(bptc)(bpmp)2]·2H2O}n (3)
The procedure is similar to that described for the synthesis of 1, except that 0.10 mmol of bpmp ligand was used instead of 0.10 mmol 4,4′-bpy. After cooling to room temperature, light orange crystals of 3 were obtained in ca. 50% yield based on Zn(NO3)2·6H2O. Anal. calcd for C50H48N8O12Zn2 (Mr = 1083.70): cal: C, 55.98%; H, 4.89%; N, 10.88%. Found: C, 55.64%; H, 4.94%; N, 10.51%. Selected IR data (KBr pellet, cm−1): 3499 (m) 3442 (m) 2812 (b) 2127 (w) 1707 (m) 1615 (s) 1410 (s) 1367 (s) 1223 (m) 1164 (m) 1135 (m) 1064 (w) 1016 (m) 839 (m) 782 (m) 724 (s) 658 (m) 575 (m) 485 (m).Synthesis of {[Zn2(bptc)(biim-4)2]·H2O}n (4)
The preparation of 4 was similar to that described procedure for the synthesis of 1, except that 0.10 mmol biim-4 was used instead of 0.10 mmol 4,4′-bpy and the reaction time was extended to 4 days. After cooling to room temperature, colorless crystals of 4 were washed by ethanol and collected in 70% yield based on Zn(NO3)2·6H2O. Anal. calcd for C36H36N8O9Zn2 (Mr = 855.47): C, 50.54%; H, 4.24%; N, 13.10%. Found: C, 50.65%; H, 4.42%; N, 12.87%. IR data (KBr pellet, cm−1): 3500 (s) 3132 (m) 3101 (m) 2941 (w) 1621 (s) 1583 (s) 1532 (m) 1344 (s) 1310 (m) 1252 (m) 1109 (s) 953 (w) 843 (w) 823 (w) 780 (m) 730 (m) 656 (m) 550 (w) 450 (w).X-ray crystallography
Single-crystal X-ray diffraction data of complexes 1–4 were collected on a Rigaku Diffractometer with a Mercury CCD area detector (Mo Kα; λ = 0.71073 Å) at room temperature. Empirical absorption corrections were applied to the data using the Crystal Clear program.16 The structures were solved by the direct method and refined by the full-matrix least-squares on F2 using the SHELXTL-97 program.17 Metal atoms in compounds were located from the E-maps and other non-hydrogen atoms were located in successive difference Fourier syntheses. The hydrogen atoms of the organic ligands were generated theoretically onto the specific atoms and refined isotropically with fixed thermal factors. Crystallographic data and structure refinement parameters for complexes 1–4 are summarized in Table S1.† Selected bond lengths and bond angles are listed in Tables S2–S6.† The CCDC numbers for complexes 1–4 are 886660–886663, respectively.Results and discussion
Synthesis and description of crystal structures
Complexes 1–4 were obtained by solvothermal reactions of H4bptc and Zn(NO3)2·6H2O together with four kinds of neutral N-donor ligands. Structural investigation reveals that these four CPs exhibit various topological architectures, which are originated from auxiliary neutral N-donor ligands with different flexibility. In addition, three kinds of coordination modes of H4bptc ligand are observed in complexes (Scheme 2). Herein, their detailed structures and luminescent properties are investigated as follows.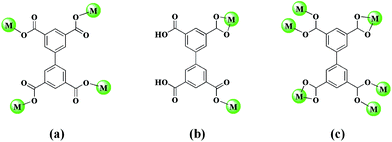 | ||
| Scheme 2 The coordination modes of H4bptc ligands in complexes 1 (a), 2 (b), 3 (c) and 4 (a), respectively. | ||
{[Zn2(bptc)(4,4′-bpy)(H2O)2]·2H2O}n (1)
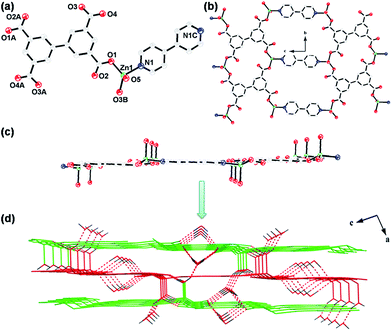
Single-crystal X-ray diffraction analysis shows that complex 1 crystallizes in the monoclinic space group P21/c and its asymmetric unit consists of one Zn(II) ion, half of a bptc4− ligand, half of a 4,4′-bpy and one coordinated water molecule. The Zn(II) center is four-coordinated by two carboxylate oxygen atoms from two fully deprotonated bptc4− ligands, one oxygen atom from one coordinated water molecule and one nitrogen atom from the 4,4′-bpy to give a distorted tetrahedral coordination geometry (Fig. 1a). The bptc4− carboxylate anions displays the κ1-κ1-κ1-κ1-μ4 coordination mode (Scheme 2a). Each Zn(II) ion is connecting to six other Zn(II) centers through one rigid bridging 4,4′-bpy and two four-connected bptc4− ligands whose two phenyl rings are nearly coplanar with dihedral angle is 0.82° (Fig. S7a†), leading to the formation of a planar layer with the Zn⋯Zn separation in the range of 9.54 to 14.29 Å (Fig. 1b and c).In addition, there are two kinds of hydrogen bond interaction in the complex 1. One is existing between coordinated water molecules and carboxylate groups, while the other is between the lattice water molecules connecting to the carboxylate groups and the coordinated water molecules. Furthermore, the adjacent layers are further stacked via these strong hydrogen bond interaction into a 3D supramolecular architecture (Fig. 1d).
{[Zn(H2bptc)(bpe)]·2H2O}n (2)
By utilizing the semi-rigid N-donor bpe instead of 4,4′-bpy, an interesting 2D → 3D parallel polycatenation framework 2 was obtained. As shown in Fig. 2a, the asymmetric unit of complex 2 contains one Zn(II) ion, one H2bptc2− anion, one bpe ligand and two guest water molecules. The Zn(II) center adopts an seriously distorted trigonal bipyramidal coordination geometry and five-coordinated with three carboxylate oxygen atoms from two carboxylic groups H2bptc2− ligands, and two nitrogen atoms from two bpe ligands. It is worth noting that H4bptc ligands are half deprotonated which adopt two totally different modes to link Zn(II) centers. One of the carboxylate groups adopts monodentate mode to bind Zn(II) centers, while another is in chelating bidentate mode connecting with the Zn(II) ions (Scheme 2b).The central Zn(II) atoms are interlinked through H2bptc2− and bpe ligands to form a 4-connected 2D corrugated layered structure with a 44-sql topology.18 Within each layer, two kinds of square windows with dimensions of 12.047 × 13.332 Å and 12.047 × 13.293 Å based on the Zn⋯Zn distance are observed (Fig. 2b). Interestingly, a rather rare example of 2D → 3D framework 2 is constructed by polycatenation of these undulating sheets. Among them, each 44-sql sheet with dihedral angle 84.60° interweaves with another in parallel fashion leading to a twofold catenated network (Fig. 2c and S6†).
{[Zn2(bptc)(bpmp)2]·2H2O}n (3)
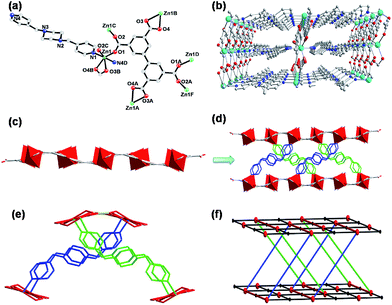
Complex 3 is crystallized in the monoclinic space group P21/c and exhibits a 3D pillared-layer framework. The asymmetric unit of 3 contains one Zn(II) ion, half of a bptc4− ligand, one bpmp molecule and one lattice water. The Zn(II) center has a slightly distorted octahedral coordination environment [ZnO4N2] defined by two nitrogen atoms from two bpmp ligands and two oxygen atoms from one chelating carboxylate group belonging to one symmetry-related bptc4− in the equatorial plane with its syn axial positions occupied by two oxygen atoms of monodentate carboxylate groups from two other symmetry-related bptc4− ligands (Fig. 3a).All the Zn–N and Zn–O bond lengths are in the range of 2.059(2)–2.249(2) Å and 2.126(2)–2.140(2) Å, respectively. The octahedral Zn(II) centers in 3 are aggregated to dinuclear zinc clusters by the carboxylate groups of bptc4− ligands. Both phenyl rings of each bptc4− ligand in complex 3 are almost parallel with dihedral angle value of 0° (Fig. S7c†). Meanwhile, the dinuclear zinc clusters are combined together by the planar bptc4− ligands to fabricate a 2D network in a × c plane (Fig. 3b). Interestingly, due to two different kinds of orientations of bpmp ligands between two adjacent layers viewed along b axis, the 3D pillared-layer structure was achieved with intrinsic self-penetration phenomenon (Fig. 3c and d).
From the topological point of view, all bptc4− ligands can be regarded as four-connected nodes and the dinuclear zinc units can be considered as six-connected nodes (Fig. 3e). As a consequence, the 3D framework 3 can be rationalized as a binodal (4,6)-connected topological network with the Schlafli symbol of {44·610·8}{44·62} (Fig. 3f).
{[Zn2(bptc)(biim-4)2]·H2O}n (4)
Single-crystal X-ray analysis reveals that complex 4 crystallizes in the monoclinic system with space group P21/c. There are two Zn(II) ions, a bptc-4 ligand, two biim-4 ligands and one lattice water in the asymmetric unit of 4 (Fig. 4a). Both Zn1 and Zn2 centers adopt tetrahedral coordination geometry in which two carboxylate oxygen atoms come from two individual fully deprotonated bptc4− ligands and two nitrogen atoms are from two individual biim-4 ligands with Zn–O and Zn–N bond lengths in the range of in 1.9477(1)–1.9743 Å and 2.0011(2)–2.0303 Å, respectively (Scheme 2a).As shown in Fig. 4c, the linear biim-4 ligands and four-connected bptc−4 ligands join the Zn(II) ions together to form left-handed and right-handed 21 helical chains, where the distances between two closest Zn(II) ions are about 9.82 Å. With a pitch of 9.536(2) Å, the helical chain winding around a twofold screw axis run parallel to the b axis, which forms a hexagonal channel (Fig. 4d). In addition, these channels are co-connected by biim-4 and bptc4− ligands to extend into a 3D framework with dimensions of 13.887(1) × 33.797(3) Å in which two phenyl rings are nearly coplanar with dihedral angle is 19.17° (Fig. 4b and S7d†). Furthermore, due to the large voids of the channels in such an array, there are three independent frameworks related to other frameworks by symmetry to constitute the final interpenetrating 3D complex 4 (Fig. 4e). On the basis of the concept of topology, all Zn(II) centers and bptc4− ligands in complex 4 both can be treated as four-connected nodes. Accordingly, this network can be regarded as a binodal (4,4)-connected network with Schlafli symbol of {4·64·8}{42·62·82} (Fig. 4e).
The effects of auxiliary N-donor ligands
Single crystals 1–4 were obtained by solvothermal reactions of H4bptc and Zn(NO3)2·6H2O together with four kinds of neutral N-donor ligands. Structural investigation reveals that these four coordination polymers present various topological supramolecular architectures originated from auxiliary neutral N-donor ligands with different flexibility (Fig. 5). | ||
| Fig. 5 Compared the distance of the four different flexible N-donor ligands (thermal ellipsoids are drawn at the 50% probability level). | ||
4,4′-bpy generally acts as a very rigid linear bridging N-donor ligand, of which two pyridyl rings maintain a nearly coplanar conformation and two N atoms with the distance of 7.0875 Å (Fig. 5a). The rigid bridging 4,4′-bpy ligand and four-connected bptc4− ligand are connected together with Zn(II) centers, leading to the rigid layers in complex 1.
The bpe ligand contains two methylene groups amid two pyridyl rings, which exhibits a relatively flexible molecular structure and two N atoms with the distance of 9.2489 Å (Fig. 5b). In complex 2, these long bpe and half-deprotonated H2bptc2− ligands are combined with Zn(II) centers to build undulating 44-sql sheets with two kinds of windows, which are large enough to let semi-rigid bpe ligands penetrate through, resulting in a parallel polycatenation framework 2.
The bpmp ligand is similar to the structural flexibility of semi-rigid bpe ligands with the distance value of two N atoms is 12.4809 Å (Fig. 5c). In addition, the piperazine rings are structurally too big to penetrate through the windows that formed by 4-connected bptc4− ligands and topologically 6-connected dinuclear Zn(II) secondary building units (SBUs). Although no polycatenation phenomenon is observed, a 3D pillar-layered framework can be achieved by the way layers are bolstered with these bpmp ligands acting as pillars. Moreover, due to two orientations of semi-rigid bpmp ligands lying between layers viewed in [0.0.1] direction, we get an attractive self-penetrating coordination framework 3.
Finally, we employed a very flexible N-donor biim-4 containing a more flexible carbon chain with the distance value of two N atoms is 10.4495 Å (Fig. 5d), which together with fully deprotonated bptc4− ligands and Zn(II) centers form hexagonal channels of huge dimension, which are so large that enable two other independent symmetry-related frameworks to penetrate through inducing a unique threefold interpenetrating coordination polymer 4.
Based on these observations, the flexibility of auxiliary neutral N-donor ligands play an important role in the self-assembly of various topological supramolecular architectures. It is undoubtedly significant for the further research of the effects on auxiliary N-donor ligands in coordination polymers.
PXRD patterns and thermal properties
The powder X-ray diffractions (PXRD) for complexes 1–4 were performed to characterize their purity and obtained at room temperature (Fig. S1†). All the diffraction peak positions on the curves correspond well with the simulated XRD patterns, indicating the phase purity of the as-synthesized samples. Thermogravimetric analysis (TGA) were performed in the temperature range of 30–800 °C under a flow of nitrogen with heating rate of 10 °C min−1 (Fig. 6). The TG curve of 1 can be divided into two stages. The first weight loss of 10.87% from 30 to 340 °C corresponds to the loss of one lattice water molecules and one coordinated water molecule (calcd 10.52%, Fig. S2†). Then, a distinct weight loss of 36.70% in the temperature range 340–540 °C, which can be assigned to the partial decomposition of the linkers in complex 1. After that, the remaining framework of 1 collapses slowly. The TG curve of 2 shows that it is stable up to 100 °C and then a weight loss of 5.87% is found between 100 to 340 °C, which is in agreement with the departure of two lattice water molecules (calcd 5.88%, Fig. S3†). Then it displays a sharp drop in the temperature range of 340–580 °C, indicating the framework of complex 2 decomposes rapidly. The TG curve of 3 shows a weight loss of 4.40% from 30 to 270 °C corresponding to the loss of one lattice water molecule (calcd 3.50%, Fig. S4†). The remaining framework starts to collapse quickly with 63.60% weight losing due to decomposition of linkers in complex 3 from 270 to 560 °C. Finally, the remaining framework breaks down gradually and completely to 800 °C. As for 4, the first weight loss of 2.22% happens in the range of 30 to 330 °C, which can be attributed to the loss of one lattice water molecule (calcd 2.10%, Fig. S5†). After that, the framework of 4 decomposes gradually in the range of 330 to 800 °C.Photoluminescence properties
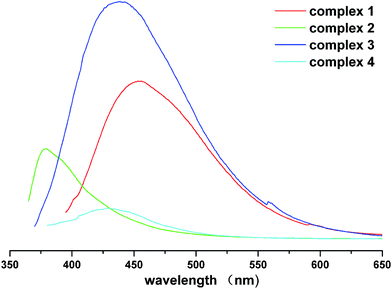
Luminescent measurements of complex 1–4 and all the free ligand were carried out in the solid state at room temperature. These solid-state luminescent spectra of 1–4 are depicted in the Fig. 7 and Table S6.† Complex 1 displays an intense fluorescent emission at 456 nm (λex = 366 nm). Excitation at 335 nm leads to an emission band with the maximum at 378 nm for 2. Complex 3 displays an intense emission at 438 nm (λex = 347 nm). The emission spectrum of complex 4 displays a very weak emission band centered at 428 nm with excitation maximum at 340 nm. The free H4bptc ligand displays a luminescent emission maximum at 385 nm in the solid state at ambient temperature. Fluorescent analyses indicate that the emission peaks of complex 1, complex 3 and complex 4 exhibit the red shift phenomenon of their emission wavelengths compared their free ligands, while complex 2 shows the blue shift phenomenon. In complex 1, the big conjugation system is derived from the bptc4− ligands whose two phenyl rings are the nearly coplanar with dihedral angle is 0.82° and 4,4′-bpy ligands, which causes a red shift of the emission wavelength. As for complex 2, two phenyl rings of the bptc4− ligands with large dihedral angle value of 56.11° and the flexible bpe ligand both have the stereospecific blockade, which destroys the conjugation system, leading to a blue shift phenomenon with the weak luminescence intensity. Both phenyl rings of each bptc4− ligand in complex 3 are almost parallel with dihedral angle value of 0°. Its weak conjugation system of trans-bpmp ligands provides the auxiliary effect, which results in a large red shift phenomenon with the stronger luminescence intensity. In complex 4, two phenyl rings bptc4− ligands are nearly coplanar with dihedral angle is 19.17° and the very flexible N-donor biim-4 with the stereospecific blockade, which causes a small red shift phenomenon with the weaker luminescence intensity. Therefore, luminescence mechanisms of complexes 1–4 should be assigned to the π–π* fluorescent emissions of all the ligand coordinated to Zn(II) centers and their differences are due to a consequence of the rotation of two phenyl rings of H4bptc ligand and the effect of N-donor ligands.19,20Conclusions
In summary, we have successfully synthesized and characterized four Zn(II)-based coordination polymers with H4bptc and four different flexibility of N-donor ligands. They display different structural and topological architectures including a 3D supramolecular polymer, a parallel polycatenated architecture, a (4,6)-connected 3D self-penetrating network and a three-fold (4,4)-connected 3D interpenetrating framework. Obviously, these various structures and diverse topologies of complexes 1–4 could be derived from the different flexibility of auxiliary N-donor ligands. These results may provide an useful guidance for further design and construction of intriguing topological supramolecular architectures based on multicarboxylate ligands and neutral N-donor ligands with different flexibility in the future.Notes and references
- (a) Z. Bao, S. Alnemrat, L. Yu, I. Vasiliev, Q. Ren, X. Lu and S. Deng, Langmuir, 2011, 27, 13554–13562 CrossRef CAS PubMed; (b) J. Liu, J. Tian and B. P. McGrail, J. Phys. Chem. C, 2012, 116, 9575–9581 CrossRef CAS; (c) Z. Lu, H. Xing, R. Sun, J. Bai and B. Zheng, Cryst. Growth Des., 2012, 12, 1081–1084 CrossRef CAS; (d) T. M. McDonald, W. R. Lee, J. A. Mason, B. M. Wiers, C. S. Hong and J. R. Long, J. Am. Chem. Soc., 2012, 134, 7056 CrossRef CAS PubMed.
- (a) W. G. Lu, D. Q. Yuan, T. A. Makal, J. R. Li and H. C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 1580 CrossRef CAS PubMed; (b) Y. B. He, S. C. Xiang, Z. J. Zhang, S. S. Xiong, C. D. Wu, W. Zhou, T. Yildirim, R. Krishna and B. L. Chen, J. Mater. Chem. A, 2013, 1, 2543 RSC; (c) Y. Takashima, V. Martinez, S. Furukawa, M. Kondo, S. Shimomura, H. Uehara, M. Nakahama, K. Sugimoto and S. Kitagawa, Nat. Commun., 2011, 2, 168 CrossRef PubMed; (d) P. Wang, J. P. Ma, Y. B. Dong and R. Q. Huang, J. Am. Chem. Soc., 2007, 129, 10620–10621 CrossRef CAS PubMed; (e) Y. H. Fu, D. R. Sun, M. Qin, R. K. Huang and Z. H. Li, RSC Adv., 2012, 2, 3309–3311 RSC.
- (a) F. Vermoortele, M. Vandichel, B. Van de Voorde, R. Ameloot, M. Waroquier, V. Van Speybroeck and D. E. De Vos, Angew. Chem., Int. Ed, 2012, 51, 4887–4890 CrossRef CAS PubMed; (b) L. J. Shen, L. J. Huang, S. J. Liang and L. Wu, RSC Adv., 2014, 4, 2546–2549 RSC; (c) Q. B. Bo, H. Y. Wang, J. L. Miao and D. Q. Wang, RSC Adv., 2012, 2, 11650–11652 RSC.
- (a) T. F. Liu, W. Zhang, W. H. Sun and R. Cao, Inorg. Chem., 2011, 50, 5242–5248 CrossRef CAS PubMed; (b) M. Hashimoto, S. Igawa, M. Yashima, I. Kawata, M. Hoshino and M. Osawa, J. Am. Chem. Soc., 2011, 133, 10348–10351 CrossRef CAS PubMed; (c) H. M. Yang, X. L. Song, T. L. Yang, Z. H. Liang, C. M. Fan and X. G. Hao, RSC Adv., 2014, 4, 15720–15726 RSC; (d) L. L. Liu, X. Zhang, S. M. Rang, Y. Yang, X. P. Dai, J. S. Gao, C. M. Xu and J. He, RSC Adv., 2014, 4, 13093–13107 RSC.
- (a) Z. Yan, M. Li, H. L. Gao, X. C. Huang and D. Li, Chem. Commun., 2012, 48, 3960–3962 RSC; (b) M. Kurmoo, Chem. Soc. Rev., 2009, 38, 1353–1379 RSC; (c) X. Y. Wang, Z. M. Wang and S. Gao, Chem. Commun., 2007, 1127–1129 RSC; (d) Q. P. Li, J. J. Qian, C. B. Tian and S. W. Du, Dalton Trans., 2014, 43, 3238–3243 RSC.
- (a) S. T. Zheng, T. Wu, C. T. Chou, A. Fuhr, P. Y. Feng and X. H. Bu, J. Am. Chem. Soc., 2012, 134, 4517–4520 CrossRef CAS PubMed; (b) W. Morris, B. Leung, H. Furukawa, O. K. Yaghi, N. He, H. Hayashi, Y. Houndonougbo, M. Asta, B. B. Laird and O. M. Yaghi, J. Am. Chem. Soc., 2010, 132, 11006–11008 CrossRef CAS PubMed.
- (a) Q. Sun, Y. Q. Wang, A. L. Cheng, K. Wang and E. Q. Gao, Cryst. Growth Des., 2012, 12, 2234–2241 CrossRef CAS; (b) F. G. Wu, J. S. Yu, S. F. Sun, H. Y. Sun, J. J. Luo and Z. W. Yu, Langmuir, 2012, 28, 7350–7359 CrossRef CAS PubMed; (c) B. Xu, Z. Lin, L. Han and R. Cao, CrystEngComm, 2011, 13, 440–443 RSC; (d) Y. Xu, P. K. Chen, Y. X. Che and J. M. Zheng, Eur. J. Inorg. Chem., 2010, 5478–5483 CrossRef CAS.
- (a) Z. Jian, D. Cui and Z. Hou, Chem.–Eur. J., 2012, 18, 2674–2684 CrossRef CAS PubMed; (b) H. Zhang, N. Li, C. Tian, T. Liu, F. Du, P. Lin, Z. Li and S. Du, Cryst. Growth Des., 2012, 12, 670–678 CrossRef CAS; (c) F. Wang, Z. S. Liu, H. Yang, Y. X. Tan and J. Zhang, Angew. Chem., Int. Ed., 2011, 50, 450–453 CrossRef CAS PubMed; (d) Y. B. Chen, Y. Kang and J. Zhang, Chem. Commun., 2010, 46, 3182–3184 RSC; (e) S. Yang, X. Lin, A. J. Blake, K. M. Thomas, P. Hubberstey, N. R. Champness and M. Schroder, Chem. Commun., 2008, 6108–6110 RSC.
- (a) W. Lu, D. Yuan, T. A. Makal, J. R. Li and H. C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 1580–1584 CrossRef CAS PubMed; (b) S. Q. Zhang, F. L. Jiang, M. Y. Wu, J. Ma, Y. Bu and M. C. Hong, Cryst. Growth Des., 2012, 12, 1452–1463 CrossRef CAS; (c) J. Ma, F. L. Jiang, L. Chen, M. Y. Wu, S. Q. Zhang, D. Han, R. Feng and M. C. Hong, Cryst. Growth Des., 2011, 11, 3273–3281 CrossRef CAS; (d) D. Han, F. L. Jiang, M. Y. Wu, L. Chen, Q. H. Chen and M. C. Hong, Chem. Commun., 2011, 47, 9861–9863 RSC; (e) T. K. Prasad, D. H. Hong and M. P. Suh, Chem.–Eur. J., 2010, 16, 14043–14050 CrossRef CAS PubMed; (f) J. Jia, M. Shao, T. Jia, S. Zhu, Y. Zhao, F. Xing and M. Li, CrystEngComm, 2010, 12, 1548–1561 RSC; (g) D. F. Sun, Y. X. Ke, T. M. Mattox, B. A. Ooro and H. C. Zhou, Chem. Commun., 2005, 5447–5449 RSC.
- (a) H. Furukawa, Y. B. Go, N. Ko, Y. K. Park, F. J. Uribe-Romo, J. Kim, M. O'Keeffe and O. M. Yaghi, Inorg. Chem., 2011, 50, 9147–9152 CrossRef CAS PubMed; (b) K. Sumida, M. R. Hill, S. Horike, A. Dailly and J. R. Long, J. Am. Chem. Soc., 2009, 131, 15120–15121 CrossRef CAS PubMed; (c) X. Lin, I. Telepeni, A. J. Blake, A. Dailly, C. M. Brown, J. M. Simmons, M. Zoppi, G. S. Walker, K. M. Thomas, T. J. Mays, P. Hubberstey, N. R. Champness and M. Schroeder, J. Am. Chem. Soc., 2009, 131, 2159–2171 CrossRef CAS PubMed; (d) J. J. Qian, F. L. Jiang, D. Q. Yuan, M. Y. Wu and M. C. Hong, Chem. Commun., 2012, 48, 9696–9698 RSC; (e) H. B. Zhang, P. Lin, X. C. Shan, Q. P. Li and S. W. Du, Chem. Commun., 2013, 49, 2231–2233 RSC.
- (a) S. T. Zheng, T. Wu, F. Zuo, C. Chou, P. Feng and X. Bu, J. Am. Chem. Soc., 2012, 134, 1934–1937 CrossRef CAS PubMed; (b) D. Banerjee, S. J. Kim, H. Wu, W. Xu, L. A. Borkowski, J. Li and J. B. Parise, Inorg. Chem., 2011, 50, 208–212 CrossRef CAS PubMed; (c) Z. Chang, D. S. Zhang, T. L. Hu and X. H. Bu, Cryst. Growth Des., 2011, 11, 2050–2053 CrossRef CAS; (d) J. J. Qian, F. L. Jian, L. J. Zhang, K. Z. Su, J. Pan, Q. P. Li, D. Q. Yuan and M. C. Hong, Chem. Commun., 2014, 1678–1681 RSC.
- (a) H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. O. Yazaydin, R. Q. Snurr, M. O'Keeffe, J. Kim and O. M. Yaghi, Science, 2010, 329, 424–428 CrossRef CAS PubMed; (b) C. J. Doonan, W. Morris, H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 9492–9493 CrossRef CAS PubMed; (c) B. L. Chen, M. Eddaoudi, S. T. Hyde, M. O'Keeffe and O. M. Yaghi, Science, 2001, 291, 1021–1023 CrossRef CAS PubMed; (d) J. J. Qian, F. L. Jiang, K. Z. Su, L. J. Zhang, X. J. Li, D. Q. Yuan and M. C. Hong, J. Mater. Chem. A, 2013, 1, 10631–10634 RSC.
- (a) J. Zhao, D. S. Li, X. J. Ke, B. Liu, K. Zou and H. M. Hu, Dalton Trans., 2012, 41, 2560–2563 RSC; (b) D. S. Li, P. Zhang, J. Zhao, Z. F. Fang, M. Du, K. Zou and Y. Q. Mu, Cryst. Growth Des., 2012, 12, 1697–1702 CrossRef CAS; (c) H. Wu, J. Yang, Z. M. Su, S. R. Batten and J. F. Ma, J. Am. Chem. Soc., 2011, 133, 11406–11409 CrossRef CAS PubMed; (d) Y. Pang, D. Tian, X. F. Zhu, Y. H. Luo, X. Zheng and H. Zhang, CrystEngComm, 2011, 13, 5142–5151 RSC.
- (a) R. Feng, L. Chen, Q. H. Chen, X. C. Shan, Y. L. Gai, F. L. Jiang and M. C. Hong, Cryst. Growth Des., 2011, 11, 1705–1712 CrossRef CAS; (b) X. L. Li, G. Z. Liu, L. Y. Xin and L. Y. Wang, CrystEngComm, 2012, 14, 1729–1736 RSC; (c) Y. Li, L. Shi, L. X. Qin, L. L. Qu, C. Jing, M. Lan, T. D. James and Y. T. Long, Chem. Commun., 2011, 47, 4361–4363 RSC; (d) H. Wu, J. Yang, Z. M. Su, S. R. Batten and J. F. Ma, J. Am. Chem. Soc., 2011, 133, 11406–11409 CrossRef CAS PubMed; (e) X. F. Zhang, W. C. Song, Q. Yang and X. H. Bu, Dalton Trans., 2012, 41, 4217–4223 RSC.
- (a) J. J. Wang, P. X. Cao and L. J. Gao, Chin. J. Struct. Chem., 2011, 30, 1787 CAS; (b) X. T. Zhang, L. M. Fan, Z. Sun and J. M. Dou, Cryst. Growth Des., 2013, 13, 792 CrossRef CAS; (c) F. L. Yang, Q. S. Zheng, Z. X. Chen and Y. M. Zhou, CrystEngComm, 2013, 15, 7031 RSC.
- CrystalClear, version 1.36, Molecular Structure Corp. and Rigaku Corp., The Woodlands, TX, and Tokyo, Japan, 2000 Search PubMed.
- G. M. Sheldrick, SHELXS 97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, Germany Search PubMed.
- V. A. Blatov, M. O'Keeffe and D. M. Proserpio, CrystEngComm, 2010, 12, 44–48 RSC.
- J. J. Wang, L. Gou, H. M. Hu, Z. X. Han, D. S. Li, G. L. Xue, M. L. Yang and Q. Z. Shi, Cryst. Growth Des., 2007, 7, 1514–1521 CAS.
- M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houka, J. Am. Chem. Soc., 2009, 38, 1330 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, crystallographic information, additional figures, IR, TGA and XRD pattern. CCDC: 886660–886663 for complexes 1–4, respectively. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra04505j |
| This journal is © The Royal Society of Chemistry 2014 |