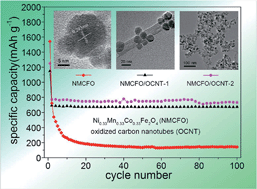
We report a solvothermal synthesis of Ni0.33Mn0.33Co0.33Fe2O4 (NMCFO) nanoparticles anchored on the surface of oxidized carbon nanotubes (OCNT) to form NMCFO/OCNT nanocomposites as advanced anode materials in Li-ion batteries. Ni(CH3COO)2, Mn(CH3COO)2, Co(CH3COO)2, and FeCl3 were employed as the metal precursors and CH3COONa, HOCH2CH2OH and H2O as the mixed solvent in the synthesis. The obtained samples were characterized by X-ray diffraction, thermogravimetric analysis, inductively coupled plasma optical emission spectrometry, X-ray photoelectron spectroscopy, transmission electron microscopy, and scanning electron microscopy. It is found that the OCNT provided functional groups on the outer walls to nucleate and anchor NMCFO nanoparticles (5–20 nm), while retaining the inner walls intact with high conductivity. Compared with the bare NMCFO nanoparticles, NMCFO/OCNT composites showed a significantly improved electrochemical performance because OCNT can substantially inhibit the aggregation of NMCFO nanoparticles and buffer the volume change, and moreover, the inner walls of OCNT provide excellent electronic conduction pathways. This work opens an effective way for the fabrication of ferrite-based metal oxide/OCNT hybrids as promising anode materials for Li-ion batteries.