Synthesis and thermomechanical property study of Novolac phenol-hydroxymethyl furfural (PHMF) resin
Zhongshun Yuan†
*,
Yongsheng Zhang† and
Chunbao (Charles) Xu*
Institute for Chemicals and Fuels from Alternative Resources (ICFAR), Department of Chemical and Biochemical Engineering, Western University, London, ON N6GA5B9, Canada. E-mail: cxu6@uwo.ca; zyuan25@uwo.ca
First published on 3rd July 2014
Abstract
Phenol-5-hydroxymethyl furfural resins were synthesized by reacting phenol with 5-hydroxymethyl furfural (HMF) generated in situ from glucose at 120 °C in the presence of CrCl2/CrCl3 and tetraethylammonium chloride (TEAC) catalysts. The phenol–HMF (PHMF) resin was found to have a relative weight average molecular weight of 700–900 g mol−1. The resins have a structure similar to Novolac phenol–formaldehyde (PF) resin, suggesting that the PHMF resins synthesized using renewable resources may be a promising formaldehyde-free alternative to conventional Novolac PF resins. The PHMF resin was utilized as polymer matrix in fibreglass reinforced plastic (FRP) composites and demonstrated to have similar or better tensile strength than the FRP with conventional PF resin.
1. Introduction
Phenol–formaldehyde (PF) resin was the first commercialized synthetic resin with wide applications in coatings, adhesives, casting, engineering materials, and household products. However, the discovery of the carcinogenic effects of formaldehyde1 and more stringent environmental regulations to reduce volatile organic compounds (VOCs) in the last decade have exerted increasing pressure on the applications of PF resins due to unavoidable emission of formaldehyde during product processing and off-gassing from the final application.Glucose is the main building block of cellulose, hemicellulose, and starch and the most abundant renewable fixed carbon source in nature. With the projected depletion of fossil resources fast approaching, glucose could be one of the future sustainable and renewable carbon sources for fuels (bio-ethanol, bio-butanol, dimethylfuran, etc.) and chemicals after certain chemical conversions. The transformation of glucose to HMF, a platform chemical, under the catalysis of several transition metals, has been demonstrated in water, organic solvents, and ionic liquids.2–7 Among a variety of metals being tested, Zr and Cr are found to be very effective to catalyze the transformation. In the presence of SO4/ZrO2 and SO4/ZrO2–Al2O3 catalysts, glucose can be converted into HMF with 48% yield in water solution.2 Zhao et al.3 first found that chromium chlorides (CrCl2, CrCl3) combined with alkyl-imidazolium chloride ionic liquids (RMIMCl) could catalyze the conversion at a high yield up to 69%. Since then, extensive research was conducted for the conversion of glucose,4,5,7 cellulose and biomass6 to HMF with CrCl2/CrCl3–RMIMCl catalyst systems. Ying, et al., achieved 80% HMF yield by treating glucose with N-heterocyclic carbine combined with RMIMCl–CrCl2 catalyst.4 Li et al.5 obtained 91% conversion of glucose into HMF using Zhao's catalyst systems under microwave irradiation. Binder6 et al. demonstrated that quaternary ammonium halo and alkaline metal halo (Cl, Br) salts in a polar aprotic organic solvent can replace ionic liquids as the co-catalyst in the chromium chloride catalyzed conversion of glucose to HMF with yields as high as 80% at optimal condition. Realizing the unfavorable effect of water (the reaction by-product) on the decomposition of HMF to side product, Valente7 et al. significantly improved HMF yields to 91% by using CrCl3/RMIMCl–toluene biphase system to extract HMF from ionic liquid into toluene phase.
The structure of HMF is a combination of furfural and hydroxymethyl furan, and furfural is known to react with phenol under both basic8,9 and acidic10 conditions to form phenolic polymers. Therefore, it is speculated that HMF can replace formaldehyde to synthesize phenol–HMF (PHMF) resin – a green alternative to PF resins, which has not been reported in the open literature.
HMF is thermally labile under long term heating at both acidic and basic conditions,7 therefore, the separation of HMF via distillation presents a significant challenge. In this work, we designed a creative one-pot approach to synthesize PHMF resin by reacting phenol with HMF in situ generated from glucose in the presence of CrCl2/CrCl3/TEAC (tetraethylammonium chloride) catalysts. Here CrClx catalyze both the reaction of HMF formation and its resinification with phenol. The PHMF resin were characterized by GPC (gel permeation chromatography) for its molecular weight and distribution, differential scanning calorimetry (DSC) for curing behavior, and Fourier Transform Infrared Spectroscopy (FTIR) and 1H and 13C nuclear magnetic resonance (NMR) for chemical structure. Moreover, fibreglass reinforced plastic (FRP) composites were prepared using the PHMF resin and conventional phenol formaldehyde resin (PF) as a reference, and the tensile strengths of the FRP samples were measured.
2. Experimental
2.1. Materials
Reagent grade phenol (99.0%), CrCl2 (95.0%), CrCl3·6H2O (98.0%), D-glucose (99.5%), tetraethyl ammonium chloride hydrate (99.0%), hexamethylenetetramine (HMTA), d6-DMSO and 5-hydroxymethyl furfural (99.0%) were purchased from Sigma-Aldrich. Tetrahydrofuran (THF, HPLC grade), formaldehyde (36 wt%) and 0.005 M H2SO4 water (HPLC grade) were obtained from Caledon Laboratories. All reagents were used as is without further purification. BGF fibreglass cloth was purchased from Freeman, Ohio.2.2. The synthesis of PHMF resins
The synthesis of phenol–HMF (PHMF) resin was first performed at atmospheric pressure in a 100 mL three-neck reactor equipped with a condenser and nitrogen outlet in the middle neck, a nitrogen inlet and a thermometer in two side necks respectively. In a typical run, the reactor was purged with nitrogen, then 9.41 g (0.100 mol) phenol, 16.20 g (0.090 mol) glucose, 0.0610 g CrCl2 (about 0.02 M in reaction mixture), 0.0570 g CrCl3·6H2O (0.01 M), and 0.1640 g (0.06 M) tetraethyl ammonium chloride (TEAC) were added subsequently. The reactor was immersed in an oil bath preheated to 120 °C and stirred with a magnetic stirrer under nitrogen atmosphere for 3 hours. For comparison, PHMF resin was also synthesized using reagent grade HMF at a HMF–phenol ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 under conditions similar to those described above. Resins synthesis at higher glucose to phenol ratios, was conducted in a 100 mL ACE glass pressure reactor. Typically, 7.05 g phenol (0.075 mol), 27.0 g (0.15 mol) glucose, 0.100 g CrCl2 (0.02 M), 0.0940 g CrCl3·6H2O (0.01 M), and 0.300 g (0.06 M) TEAC, and 3.50 g water (10 wt%) were added to the reactor. The reactor was evacuated and purged with nitrogen through a rubber septum, then capped with a Teflon stopper. The reactor was placed into an oil bath preheated to 120 °C and stirred with a magnetic stirrer for 5–8 hours. After cooling, the reaction mixture was diluted with 80/20 (v/v) methanol–water to form a homogeneous solution. Samples of this solution were then taken and further diluted with HPLC solvent for glucose, phenol, and HMF quantity analysis. The product was purified (to remove unreacted glucose and phenol) by first removing the solvent using a rotary evaporator, then dissolving the remaining material in acetone, and precipitating PHMF in 90/10 (v/v) water–methanol. After vacuum drying (to completely remove water in the samples), the precipitation process resulted in a semi-solid black PHMF product, subject to carious analyses and application.
0.9 under conditions similar to those described above. Resins synthesis at higher glucose to phenol ratios, was conducted in a 100 mL ACE glass pressure reactor. Typically, 7.05 g phenol (0.075 mol), 27.0 g (0.15 mol) glucose, 0.100 g CrCl2 (0.02 M), 0.0940 g CrCl3·6H2O (0.01 M), and 0.300 g (0.06 M) TEAC, and 3.50 g water (10 wt%) were added to the reactor. The reactor was evacuated and purged with nitrogen through a rubber septum, then capped with a Teflon stopper. The reactor was placed into an oil bath preheated to 120 °C and stirred with a magnetic stirrer for 5–8 hours. After cooling, the reaction mixture was diluted with 80/20 (v/v) methanol–water to form a homogeneous solution. Samples of this solution were then taken and further diluted with HPLC solvent for glucose, phenol, and HMF quantity analysis. The product was purified (to remove unreacted glucose and phenol) by first removing the solvent using a rotary evaporator, then dissolving the remaining material in acetone, and precipitating PHMF in 90/10 (v/v) water–methanol. After vacuum drying (to completely remove water in the samples), the precipitation process resulted in a semi-solid black PHMF product, subject to carious analyses and application.
2.3. The synthesis of PF resin
As a comparison reference for PHMF property test, a conventional PF Novolac resin was prepared (85 °C, 2 h) in phenol-to-formaldehyde molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.85, using HCl (0.5 wt% of phenol) as catalyst.
0.85, using HCl (0.5 wt% of phenol) as catalyst.
2.4. Analytical methods
HPLC and GPC (high performance liquid chromatography–gel permeation chromatography) analysis were conducted with a Waters Breeze instrument (1525 binary pump with refractive index (RI) and UV detectors). Reaction mixture was diluted with HPLC water and filtrated to get a clear solution. Glucose, phenol, and HMF contents were analyzed by HPLC equipped with a Bio-Rad Aminex HPX-87H column and using 0.005 M H2SO4 HPLC grade water as the mobile phase with a flow rate of 0.6 mL min−1. Polymer molecular weights were measured using a Waters Styragel HR1 GPC column and using THF as the eluent at 1 mL min−1. Linear polystyrene standards were used for the molecular weight calibration. GC-MS analysis was conducted with an Agilent 7890B GC coupled with a 5977A MSD using a 30 m × 0.5 mm × 0.25 μm DB-5 column with temperature programing as follows: a 1 min hold at an initial temperature of 50 °C followed by a 30 °C min−1 ramp to a final temperature of 280 °C with 1 min hold. FTIR spectrum was obtained with a Nicolet 6700 Fourier Transform Infrared Spectroscopy with a smart ITR/ATR accessory which allowed direct measurement using original solid/liquid samples. 1H NMR and 13C NMR (nuclear magnetic resonance) spectra of resin dissolved in d6-DMSO were acquired at 25 °C on a Varian Inova 600 NMR spectrometer equipped with a Varian 5 mm triple-resonance indirect-detection HCX probe. Differential scanning calorimetry (DSC) (Mettler Toledo DSC 1) was used to monitor the curing behavior of homogeneous mixture of the synthesized PHMF resin and the curing agent.Carver (hydraulic unit model 3925) hot press was used for composites fabrication. ADMET Expert 7600 universal test machine was used to measure the tensile strengths of the FRP samples. Fibreglass reinforced plastic (FRP) composites were prepared by lay up method using BGF fibreglass cloth and PHMF resin or conventional phenol–formaldehyde resin (PF) with 15 wt% HMTA (mass ratio of glass fiber/resin + curing agent = 1/1) as a curing agent under a curing procedure of 120 °C for 30 min, 150 °C for 30 min, and 180 °C for 1 h under 2000 psi pressure in a hot press. The cured composites were cut into rectangular samples with the dimensions of 50 × 10 × 1 mm and were tested using a Netzsch 242C DMA. Three point bending geometry was applied at a driving frequency of 1 Hz with a deflection of 5 μm. The temperature ramped from 50 to 350 °C under at a heating rate of 5 °C min−1. The pressed composites were also shaped into dog bone samples and their tensile strengths were measured in accordance to ASTM 638.
3. Results and discussion
3.1. Preparation of glucose-based resin under ambient pressure
Inspired by Zhao's research,3 our previous study demonstrated that conversion of glucose to HMF can be achieved with an inexpensive ionic liquid TEAC.11 Zhao's and our study showed that both CrCl2 and CrCl3 are very efficient catalysts for converting glucose to HMF through fructose intermediate, and CrCl2 gave higher HMF yield than CrCl3. The synthesis of PHMF resin was first performed at 120 °C with different catalyst combination and varying glucose–phenol ratios in bulk conditions (without water). In the most of the reactions, glucose conversions were over 90%, while very low concentrations of free HMF (mostly <1.5 wt%) were detected, implying an in situ consumption of HMF after its formation via phenol–HMF resinification reaction. The phenol–HMF resinification reaction was also evidenced by the considerable conversion of phenol (36–63% depending on phenol–glucose molar ratio) and the large molecular weights (Mw of 700–800 g mol−1) of the resulted polymers–PHMF resins.Fig. 1 shows the phenol and glucose conversion at different catalyst combinations. Among the five catalyst combinations, the overall phenol–glucose conversion is highest at CrCl2/CrCl3/TEAC concentration (M/M/M) = 0.02/0.01/0.06. With either CrCl2/TEAC or CrCl3/TEAC catalyst systems, the phenol and glucose conversions were much lower than those with the CrCl2/CrCl3/TEAC catalysts, especially in the case of CrCl2/TEAC. This is because CrCl2 (although more active in the formation of HMF) is less acidic than CrCl3 and the resinification/condensation reactions between phenol and HMF require a Lewis acid catalyst. The experimental results also show TEAC as a cocatalyst can promote phenol–glucose conversion as proved in Zhao and our previous study.3,11 These works also proved that 120 °C is the optimal temperature for the conversion of glucose to HMF, therefore, the phenol–HMF synthesis in this study was carried out at this temperature.
 | ||
Fig. 1 Effects of catalyst combinations on phenol and glucose conversion. Reaction conditions: phenol–glucose = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9, at 120 °C refluxed for 3 h at atmospheric pressure under nitrogen protection. 0.9, at 120 °C refluxed for 3 h at atmospheric pressure under nitrogen protection. | ||
Fig. 2 presents the experimental results for different phenol–glucose mole ratios. Fig. 2(a) shows the bulk reaction results. At a fixed catalyst concentration of CrCl2/CrCl3/TEAC = 0.02/0.01/0.06, increasing glucose–phenol molar ratio from 0.6 to 1.5 resulted in a steady increase in the phenol conversion from 36% to 63% with the glucose conversion of over 90%. When the glucose–phenol ratio was increased above 1.5, the initial magnetic stirring was found to be very difficult due to the high melting point of glucose and its low solubility in phenol. Therefore, for glucose–phenol ratio of higher than 1.5, water was added to assist the dissolving of glucose. However, to maintain the reaction temperature of water-containing reaction medium (120 °C), a pressure reactor had to be used. Since water is a by-product of both the conversion of glucose to HMF and the condensation reaction of phenol with HMF, the addition of water to the reaction system is unfavorable for the resin synthesis. Therefore, these reactions with the presence of water (Fig. 2(b)) took longer time (5 h) to reach high glucose conversion than the experiments without water. Comparing the results between Fig. 2(a) and (b), it can be seen that phenol conversion at glucose–phenol molar ratios of 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the pressure reactor were much higher (68–92%) than those at lower glucose–phenol ratio, which suggests the reaction at a higher glucose–phenol molar ratio favors higher conversion of phenol into PHMF resins. The molecular weight of the resins (measured by GPC) also increased from 800 g mol−1 to 900 g mol−1 with the glucose–phenol mole ratio increase from 1.5 to 2.
1 in the pressure reactor were much higher (68–92%) than those at lower glucose–phenol ratio, which suggests the reaction at a higher glucose–phenol molar ratio favors higher conversion of phenol into PHMF resins. The molecular weight of the resins (measured by GPC) also increased from 800 g mol−1 to 900 g mol−1 with the glucose–phenol mole ratio increase from 1.5 to 2.
Longer reaction time was conducted for phenol–glucose mole ratio of 1/2. The positive effects of reaction time on the phenol conversion and PHMF formation reaction are directly revealed by the results presented in Fig. 3. Both phenol and glucose conversion increased from 69% to 92% and 80 to 99%, respectively, as the reaction time extended from 5 h to 8 h, accompanied by an little increase in Mw of the PHMF products. Fig. 3 and entries 3 to 5 in Table 1 show the increase in phenol conversion was more than that of glucose for extended reaction time. This is probably because the polymer chain mostly had ended with HMF (see Scheme 1) and was able to attach more phenol to the chain end.
 | ||
Fig. 3 Effects of reaction time on phenol and glucose conversion. Reaction conditions: phenol–glucose mole ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, CrCl2/CrCl3/TEAC = 0.02/0.01/0.06, 120 °C in pressure reactor. 2, CrCl2/CrCl3/TEAC = 0.02/0.01/0.06, 120 °C in pressure reactor. | ||
| Entry | Ph–Glu (mol mol−1) | H2O (g) | Time (h) | Mw g mol−1 | Conversion (%) | HMF (wt%) | |
|---|---|---|---|---|---|---|---|
| Ph | Glu | ||||||
| a Catalyst concentration: CrCl2/CrCl3/TEAC = 0.02/0.01/0.03 (M/M/M). Ph = phenol, Glu = glucose. | |||||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
6 | 5 | 700 | 67.5 | 98.7 | 1.10 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
6 | 5 | 760 | 72.1 | 89.4 | 0.67 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
6 | 5 | 760 | 69.0 | 80.4 | 1.46 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
6 | 6 | 810 | 73.0 | 93.7 | 0.75 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
6 | 8 | 900 | 91.7 | 98.9 | 0.65 |
3.2. Reaction mechanism of PHMF resin
A possible reaction mechanism for the synthesis of phenol–HMF resin is proposed in Scheme 1. In the presence of CrCl2, CrCl3 and TEAC, glucose can be isomerized to fructose, which is subsequently dehydrated (losing three molecules of water) to HMF.2–7,11 In the presence of a Lewis acid (CrCl3), the electron rich carbons of the para and ortho positions of phenol can undergo nucleophilic addition to the electrophilic aldehyde group in HMF. Under the assistance of Lewis acid, the hydroxymethyl group in HMF can also react with the para and ortho position of phenol OH through a Friedel–Crafts alkylation mechanism. The final product is a resin with a structure similar to that of branched Novolac phenolic resins, but with some of the benzene rings substituted by furan rings and some of the methylene linkages replaced by hydroxyl methylene linkages. Since furan is also an electron-rich aromatic ring, reactions between furan rings and aldehyde or hydroxymethyl groups in HMF could also occur. That is probably one of the reasons why the glucose consumption is very high.It was found12–15 that in glucose and fructose to HMF conversion, the presence of a moderate amount of water could improve HMF yield, but extra amounts of water in the reaction system promoted the decomposition of HMF into levulinic acid and decreased HMF yield. In the present system, the newly formed HMF quickly reacted with phenol after its formation, which drove the dehydration reaction forward and thus reduced HMF concentration and prevented its side reactions. This was confirmed by GC-MS analysis as there was no detectable levulinic acid. These experimental results proved the benefit of in situ one-pot reactions. However, under acidic conditions,16,17 glucose may be converted to humin, a water insoluble but organic soluble polymer of dehydrated glucose, HMF, and the degradation products of HMF. The PHMF resin may contain a few mixture of PHMF and humin or a copolymer of PHMF and humin. The existence of humin may not significantly affect the application of the PHMF product as long as it can be incorporated into cured product. The final resin was found insoluble in water, but soluble in most organic solvents including acetone and tetrahydrofuran. This proved that the resin product was not an oligomer of glucose, but rather a highly dehydrated polymeric product. The PHMF resin can be purified by dissolving the reaction mixture in acetone, then precipitating the mixture into water–methanol to remove catalysts, unreacted glucose and phenol. For phenol–glucose = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 8 h reaction, the yield after purification was 57% (weight of the product divided by feed, theoretical yield is 75%). Since the catalysts are all non-toxic, and they are also active in promoting resin curing reactions, in practice, the only purification needed for the final product is a steam distillation to recover unreacted phenol. The pH value of the final reaction mixture was measured to be about 1.0, which is almost the same as that of CrCl3 water solution, showing the acidity throughout the reaction. The presence of CrCl3 is actually beneficial as it can act as a Lewis acid catalyst for the curing reaction.
2, 8 h reaction, the yield after purification was 57% (weight of the product divided by feed, theoretical yield is 75%). Since the catalysts are all non-toxic, and they are also active in promoting resin curing reactions, in practice, the only purification needed for the final product is a steam distillation to recover unreacted phenol. The pH value of the final reaction mixture was measured to be about 1.0, which is almost the same as that of CrCl3 water solution, showing the acidity throughout the reaction. The presence of CrCl3 is actually beneficial as it can act as a Lewis acid catalyst for the curing reaction.
3.3. Characterization of glucose-based PHMF resin
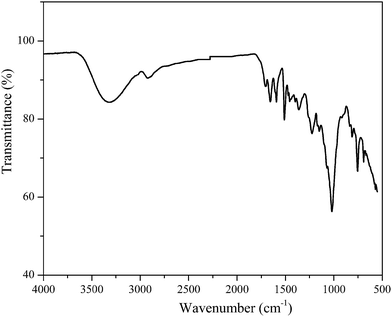
The IR spectrum of the synthesized resin (Fig. 4) reveals obvious aromatic ring structure in 1400–1600 cm−1 region, that is, carbon–carbon stretching vibrations at 1592 cm−1, 1505 cm−1, and 1450 cm−1, attributed to phenol and furan ring structures in the PHMF resins (Scheme 1). The absorptions at 1230 cm−1 and 1000 cm−1 are due to conjugated and un-conjugated C–O stretching respectively. The absorption at 748 cm−1 is due to out-of plane bending of aromatic C–H bonds. The absorption at 3275 cm−1, 2910 cm−1 and 1702 cm−1 are attributed to OH, methylene (–CH2–) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (aldehyde) stretching, respectively, which is the evidence of the condensation reaction between the aldehyde or hydroxymethyl groups in HMF and phenol para/ortho reactive sites to form PHMF –CH(OH)– and –CH2– linkages, as shown in Scheme 1.
O (aldehyde) stretching, respectively, which is the evidence of the condensation reaction between the aldehyde or hydroxymethyl groups in HMF and phenol para/ortho reactive sites to form PHMF –CH(OH)– and –CH2– linkages, as shown in Scheme 1.
In the proton NMR spectrum (Fig. 5), except for the acetone solvent peak (d6-acetone 2.0 ppm), most peaks are aromatic (6–8 ppm), resulting from the protons of phenol and HMF rings in the PHMF resin, which means the product is highly aromatic, suggesting high conversion of glucose to HMF. The peak at 9.5 ppm is the proton of the aldehyde group from incorporated HMF. The peak at 8.3 ppm is due to the hydroxyl proton of the phenol ring with hydrogen bonding. The peak at 4.0 ppm can be attributed to methylene protons.
The 13C-NMR spectra of the PHMF resin synthesized from phenol and glucose (a) and synthesized from phenol and reagent-grade HMF (b) are shown in Fig. 6. In spectrum (a), the peaks can be assigned as following: aldehyde carbon, 178 ppm; carbon adjacent to oxygen of the furan ring, 162 ppm; hydroxyl substituted phenolic carbons, 156 and 157 ppm; carbon adjacent to oxygen and aldehyde group of the furan ring, 152; carbon on phenolic ring at the meta position of OH connected carbon, 129; carbon on furan ring meta to oxygen, 119; carbon on phenolic ring at the ortho position of OH connected carbon, 115; carbon on furan ring meta to oxygen and CHO, 110; methylene and methine group, 52, 56; NMR solvent d6-dimethyl sulfoxide, 40. The remaining unidentified peaks may be ascribed to the carbons present in the glucose polymers. The two 13C-NMR spectra are very similar.
 | ||
| Fig. 6 13C-NMR. (a) PHMF resin synthesized from glucose and phenol, (b) PHMF resin synthesized from reagent HMF and phenol. | ||
Elemental analysis (C, H, and O) revealed that the purified PHMF resin at a PhOH–glucose ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 had C, H, and O contents (wt%) of 66.2, 5.5, and 27.0. The PHMF derived from reagent HMF has H, C, O contents of 70.7, 4.8, 24.3, very close to the H, C, O contents (71.3, 5.0, and 23.8 wt%) which would result from a PHMF resin composed of alternating phenol–HMF units. The 27% O content of the PHMF resin from PhOH–glucose = 1
1.5 had C, H, and O contents (wt%) of 66.2, 5.5, and 27.0. The PHMF derived from reagent HMF has H, C, O contents of 70.7, 4.8, 24.3, very close to the H, C, O contents (71.3, 5.0, and 23.8 wt%) which would result from a PHMF resin composed of alternating phenol–HMF units. The 27% O content of the PHMF resin from PhOH–glucose = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 was much lower than the feed O content of 44%, proving a significant of dehydration of glucose to HMF. The O content of PHMF was about 11% higher than that of the PHMF resin derived from reagent HMF, likely due to the over 1.0 mole ratio of HMF to phenol.
1.5 was much lower than the feed O content of 44%, proving a significant of dehydration of glucose to HMF. The O content of PHMF was about 11% higher than that of the PHMF resin derived from reagent HMF, likely due to the over 1.0 mole ratio of HMF to phenol.
3.4. Thermal behaviour of PHMF with curing agent and performances of resulted FRC
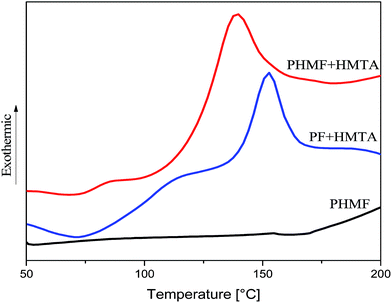
DSC (Fig. 7) was used to monitor the cure behavior of the synthesized PHMF resin. As was expected, PHMF resin can be cured by hexamethylenetetramine (HMTA) with an exothermal peak at 139 °C, lower than the curing of Novolac PF with HMTA (153 °C).18 The lower curing temperature is because metal salt Lewis acid catalyzed Novolac resin is high ortho phenol linkage resin,19 leaving para position free and the reaction of HMTA with phenol para position has lower activation energy than ortho position. For the curing of Novolac, because it is a linear or slightly branched polymer with only methylene linkage between benzene rings, its curing usually needs HMTA as curing agent. The same to Novolac PF resin, the DSC profile of PHMF resin itself has no obvious exothermal peak, showing a curing agent is needed.As Novolac resin, PHMF resin may have wide applications in refractories, friction, abrasive, felt bonding, electronics, molding, casting, photo resists, semiconductors, and composite materials where low VOCs are required. The mechanical properties of glass fiber–PHMF composite specimen cured with HMTA were investigated and the DMA profiles are elucidated in Fig. 8, where the storage modulus (E′) and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ are plotted against temperature. The Tg's of the cured samples in this study was determined by the peak temperature of tan
δ are plotted against temperature. The Tg's of the cured samples in this study was determined by the peak temperature of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (267 °C), which is a little higher but very close to the Tg of glass fiber–PF composite (250 °C),20 showing PHMF resin can even have a little higher application temperature.
δ (267 °C), which is a little higher but very close to the Tg of glass fiber–PF composite (250 °C),20 showing PHMF resin can even have a little higher application temperature.
Table 2 gives the comparison of the tensile strength of the glass fiber–PHMF with glass fiber–PF composites. Interestingly, the glass fiber–PHMF composites are likely stronger than the glass fiber–PF composites with the tensile strength results of 127 Mpa and 118 Mpa respectively.
| Sample | Tensile strength |
|---|---|
| PHMF + HMTA | 127 ± 2 MPa |
| PF + HMTA | 118 ± 1 MPa |
4. Conclusions
In summary, phenol-5-hydroxymethyl furfural (PHMF) resins were synthesized via a novel one-pot process by reacting phenol with HMF generated in situ from glucose in the presence of CrCl2/CrCl3 and tetraethylammonium chloride (TEAC) catalysts at 120 °C. The PHMF resins have a relative weight average molecular weight of 700–900 g mol−1. Similar to Novolac PF resin, the resins can be cured with HMTA with slightly lower curing temperature than PF resin. Fibreglass reinforced plastic (FRP) composites were prepared using the PHMF resin, and the tensile strength test results of the FRP samples demonstrated that the FRP composites with PHMF resin have higher tensile strength than those with conventional PF resin. Compared with conventional PF resins, the most important advantage of the PHMF resin is that carcinogenic formaldehyde is substituted with HMF derived from renewable, nontoxic, and inexpensive glucose. The PHMF resins may have great potential to replace Novolac PF resin in many applications for example as polymer matrixes in composites materials.Acknowledgements
The authors are grateful for the financial support from NSERC/FPInnovations Industrial Research Chair Program in Forest Biorefinery and the Ontario Research Fund-Research Excellence (ORF-RE) from Ministry of Economic Development and Innovation. Support from the industrial partners including FPInnovations, Arclin Canada, BioIndustrial Innovation Centre, and CENNATEK is also acknowledged. The author (C. Xu) also acknowledges the funding from NSERC via the Discovery Grant Program.References
- L. Zhang, C. Steinmaus, D. A. Eastmond, X. K. Xin and M. T. Smith, Mutat. Res., Rev. Mutat. Res., 2009, 681, 150 CrossRef CAS PubMed.
- H. Yan, Y. Yang, D. Tong, X. Xiang and C. Hu, Catal. Commun., 2009, 10, 1558 CrossRef CAS PubMed.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597 CrossRef CAS PubMed.
- G. Yong, Y. G. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2008, 47, 9345–9348 CrossRef CAS PubMed.
- C. Li, Z. Zhang and Z. K. Zhao, Tetrahedron Lett., 2009, 50, 5403 CrossRef CAS PubMed.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979 CrossRef CAS PubMed.
- S. Lima, P. Neves, M. M. Antunes, M. Pillinger, N. Ignatyev and A. A. Valente, Appl. Catal., 2009, 363, 93 CrossRef CAS PubMed.
- D. Wu and R. Fu, Microporous Mesoporous Mater., 2006, 96, 115 CrossRef CAS PubMed.
- F. B. Oliveira, C. Gardrat, C. Enjalbal, E. Frollini and A. Castellan, J. Appl. Polym. Sci., 2008, 109, 2291 CrossRef CAS.
- D. Long, J. Zhang, J. Yang, Z. Hu, T. Li, G. Cheng, R. Zhang and L. Ling, New Carbon Mater., 2008, 23, 165 CrossRef CAS.
- Z. Yuan, C. Xu, S. Cheng and M. Leitch, Carbohydr. Res., 2011, 346, 2019 CrossRef CAS PubMed.
- B. Girisuta, L. P. B. M. Janssen and H. J. Heeres, Chem. Eng. Res. Des., 2006, 84, 339 CrossRef CAS.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044 CrossRef CAS PubMed.
- K.-i. Shimizu, R. Uozumi and A. Satsuma, Catal. Commun., 2009, 10, 1849 CrossRef CAS PubMed.
- A. Fadel, R. Yefsah and J. Salaun, React. Polym., 1987, 6, 93 CAS.
- X. Hu, C. Lievens, A. Larcher and C.-Z. Li, Bioresour. Technol., 2011, 102, 10104 CrossRef CAS PubMed.
- S. J. Dee and A. T. Bell, ChemSusChem, 2011, 4, 1166 CrossRef CAS PubMed.
- E. D. Medeiros, J. M. Agnelli, K. Joseph, L. D. Carvalho and L. C. Mattoso, J. Appl. Polym. Sci., 2003, 90, 1678 CrossRef.
- J. Huang, M. Xu, Q. Ge, M. Lin, Q. g. Lin, Y. Chen, J. n. Chu, L. Dai and Y. Zou, J. Appl. Polym. Sci., 2005, 97, 652 CrossRef CAS.
- S. G. Kuzak and A. Shanmugam, J. Appl. Polym. Sci., 1998, 73, 649 CrossRef.
Footnote |
| † Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |