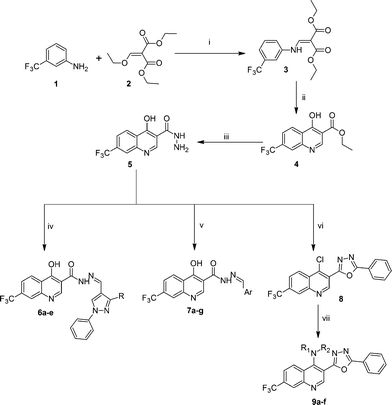
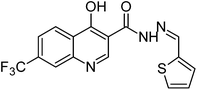
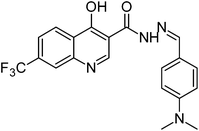
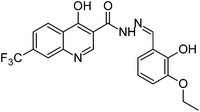
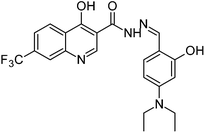
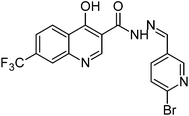
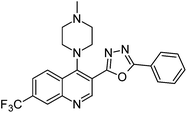
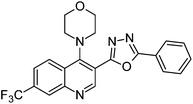
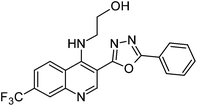
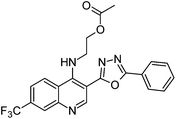
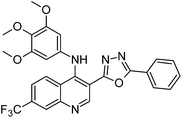
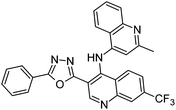
Synthesis, characterization and antimicrobial studies of some new trifluoromethyl quinoline-3-carbohydrazide and 1,3,4-oxadiazoles†
B. Garudacharia,
Arun M. Isloor*a,
M. N. Satyanarayab,
K. Anandac and
Hoong-Kun Fund
aMedicinal Chemistry Laboratory, Department of Chemistry, National Institute of Technology Karnataka, Surathkal, Mangalore-575 025, India. E-mail: isloor@yahoo.com; Fax: +91 824 2474033; Tel: +91 824 2474000
bDepartment of Physics, National Institute of Technology Karnataka, Surathkal, Mangalore-575 025, India
cBiological Sciences Division, Poornaprajna Institute of Scientific Research, Devanahalli, Bangalore-562 110, India
dDeparment of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh 11451, Saudi Arabia
First published on 26th June 2014
Abstract
The present paper describes the synthesis of two new series of 7-(trifluoromethyl)-4-hydroxy substituted quinoline carbohydrazide derivatives (6a–e and 7a–g) and N-alkyl-3-(5-phenyl-1,3,4-oxadiazol-2-yl)-7-(trifluoromethyl) quinolin-4-amine derivatives (9a–f). Newly synthesized compounds were characterized by spectral studies. The structure of 9a was evidenced by X-ray crystallographic study. Synthesized compounds were screened for their antibacterial performance against Mycobacterium smegmatis and Pseudomonas aeruginosa. Antifungal activity was also carried out on the fungal stains Candida albicans and Penicillium chrysogenum. Compounds 7a and 9c showed significant antimicrobial activity against all the tested microorganisms. Among all the compounds, 6d and 6e showed the lowest MIC value of 6.25 μg mL−1 against Mycobacterium smegmatis indicating these compounds can be possible future antituberculosis agents.
1. Introduction
Heterocyclic compounds play an important role in medicinal chemistry. They are well known to possess diverse pharmacological properties, viz. antimicrobial, anti-inflammatory, anticonvulsant, antiviral, antimalarial, antituberculosis and anticancer.1–4 Pathogenic microorganisms developing resistance to drugs is a serious problem in the last few decades. Different structural modifications have been made to enhance the antimicrobial activity by introducing different functional groups around the quinoline nucleus. Among the heterocyclic compounds, substituted quinolines are more important because of their wide spectrum of biological activity. A large variety of quinoline derivatives have been used as anticancer,5 antiviral,6 anti-inflammatory,7 antimicrobial,8,9 antioxidant,10 antimalarial,11 anti-tuberculosis,12 agents. Mefloquine (antimalarial) and bedaquiline or TMC207 (anti-tuberculosis) are well-known drugs which contain a quinoline core moiety (Fig. 1).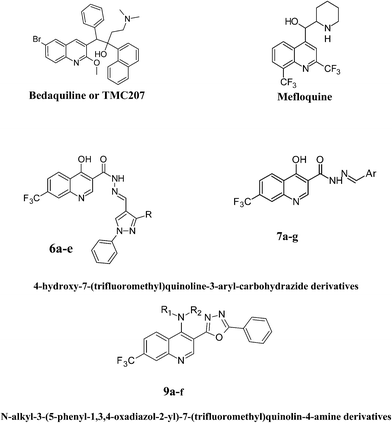 | ||
| Fig. 1 Examples of quinoline-based drugs and the outline of structural modifications on the lead compound. | ||
It has been well established that fluorinated quinolines, in particular, CF3 substituted quinolines have got a significant place in modern medicinal chemistry. Introduction of trifluoromethyl group provides better electronic effect at neighboring carbon centers, as well as having a substantial effect on the molecule's dipole moment, acidity and basicity of neighboring groups.13 Their biological studies clearly indicated that, the presence of trifluoromethyl group in position seven and eight of the quinoline ring is responsible for their enhanced biological activity14–16 and are the subject of considerable growing interest. Further, various types of hydrazones have attracted continued interest in the field of medicine owing to their varied biological activities as antimicrobial,17 antimalarial,18 and antitubercular properties.19 On the other hand, compounds containing 1,3,4-oxadizole rings are very well known to exhibit powerful antimicrobial,20,21 analgesic,22 cannabinoid receptor 2 (CB2) agonist,23 VEGFR-2 and Tublin inhibitor24 properties. Therefore there is great importance for the synthesis of oxadiazoles as target structures and evaluation of their biological activities.
In our previous studies, we reported the synthesis and antimicrobial activity of some fluorophenyl and trifluoromethyl quinoline derivatives. It was found that, fluorinated compounds are good antimicrobial agents. Encouraged by these results and in continuation of the synthesis of new heterocyclic compounds,4,25,26 the present study was focused on the synthesis of new trifluoromethylquinoline derivatives, their characterization and antimicrobial activity.
2. Results and discussion
2.1. Chemistry
The targeted compounds (6a–e, 7a–g and 9a–f) were synthesized by employing sequential reactions, which are presented in Scheme 1. The quinolone skeletons were built up by the Gould–Jacobs procedure starting from 3-(trifluoromethyl) aniline 1. Condensation of 1 with diethyl ethoxymethylene malonate and subsequent thermal cyclization in dowtherm (biphenyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : biphenyloxide (3
: biphenyloxide (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7)) yielded the 4-hydroxy-quinoline-3-carboxylic ester 4 (ref. 16 and 27) which on condensation with hydrazine hydrate in alcoholic medium resulted 4-hydroxy-7-trifluoromethyl-quinoline-3-carboxylic acid hydrazide 5.28 Further, the key intermediates, pyrazole-4-carbaldehydes were prepared by the Vilsemeier–Haack reaction of the corresponding hydrazones.29
7)) yielded the 4-hydroxy-quinoline-3-carboxylic ester 4 (ref. 16 and 27) which on condensation with hydrazine hydrate in alcoholic medium resulted 4-hydroxy-7-trifluoromethyl-quinoline-3-carboxylic acid hydrazide 5.28 Further, the key intermediates, pyrazole-4-carbaldehydes were prepared by the Vilsemeier–Haack reaction of the corresponding hydrazones.29
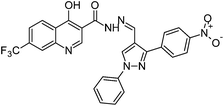
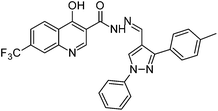
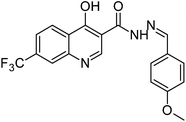
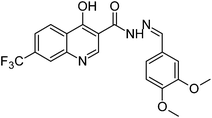
The final compounds 4-hydroxy-7-(trifluoromethyl)quinoline-3-carbohydrazide (6a–e and 7a–g) were obtained by reacting quinoline hydrazide 5 with various substituted aldehydes in ethanolic media (Scheme 1). Reaction of 5 with benzoic acid in POCl3 yielded 4-chloro-3-(5-phenyl-1,3,4-oxadiazol-2-yl)-7-(trifluoromethyl)quinoline 8. Finally chlorine in 8 were replaced with various aliphatic and aromatic amines to obtain the targeted N-alkyl-3-(5-phenyl-1,3,4-oxadiazol-2-yl)-7-(trifluoromethyl)quinolin-4-amine derivatives 9a–f. The crude products were purified by column chromatography using pet ether and ethyl acetate (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluent. All the synthesized compounds were characterized by IR, NMR, mass spectral and C, H, N elemental analysis.
3) as the eluent. All the synthesized compounds were characterized by IR, NMR, mass spectral and C, H, N elemental analysis.
Formation of 4-hydroxy-7-(trifluoromethyl)quinoline-3-carbohydrazide derivatives (6a–e and 7a–g) were confirmed by recording their IR, 1H-NMR, 13C-NMR and mass spectra. The FT-IR spectrum of compound 6a showed two bands at 3410 cm−1 and 3138 cm−1, which are due to the hydroxyl and amide groups respectively. Band at 1668 cm−1 is due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of carbonyl group. The 1H-NMR spectrum of 6a showed a singlet at δ 8.07 ppm which is due to the imine proton (N
O stretch of carbonyl group. The 1H-NMR spectrum of 6a showed a singlet at δ 8.07 ppm which is due to the imine proton (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH). Hydroxyl and amide protons appeared as singlet at δ 12.90 and 13.10 ppm respectively further confirmed the structure of the compound. The mass spectrum of 7a showed molecular ion peak at m/z = 502 (M + 1), which is in agreement with the molecular formula C27H18F3N5O2. Similarly the spectral values for all the compounds and C, H, N analyses are presented in the experimental part and the characterization data are provided in Tables 1 and 2.
CH). Hydroxyl and amide protons appeared as singlet at δ 12.90 and 13.10 ppm respectively further confirmed the structure of the compound. The mass spectrum of 7a showed molecular ion peak at m/z = 502 (M + 1), which is in agreement with the molecular formula C27H18F3N5O2. Similarly the spectral values for all the compounds and C, H, N analyses are presented in the experimental part and the characterization data are provided in Tables 1 and 2.
2.2. Antimicrobial studies
Antibacterial studies of newly synthesized compounds (6a–e, 7a–g and 9a–f) were carried out against two pathogenic bacteria Mycobacterium smegmatis (MTCC 943) and Pseudomonas aeruginosa (MTCC4676) by well diffusion method using nutrient agar media.30,31 Antifungal activity was carried out against two fungi Candida albicans (MTCC 183) and Penicillium chrysogenum (MTCC 6795).32,33 All the compounds were dissolved in dimethylsulfoxide (DMSO) and used for testing at 25 and 50 μg mL−1 concentrations. Antimicrobial activities were determined by measuring the diameter of inhibition zone in millimetre. A minimum inhibition concentration (MIC) was also determined for the test compounds at concentration ranging from 1.6–50 μg mL−1 against three bacteria M. smegmatis, P. aeruginosa and Staphylococcus aureus and one fungi C. albicans. Ciprofloxacin was used as standard antimicrobial compound for antibacterial studies, while fluconazole was used as standard for the antifungal studies. All the experiments were performed in triplicates and average value was taken. The details of the results of the antimicrobial analysis are furnished in Tables 3 and 4.| Zone of inhibition in mm (mean ± S.D.) n = 3 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound no. | Mycobacterium smegmatis | Pseudomonas aeruginosa | Candida albicans | Penicillium chrysogenum | ||||
| a AB; anti-bacterial standard ciprofloxacin; AF; anti-fungal standard fluconazole; —: not detected inhibition; control; dimethylsulfoxide. | ||||||||
| Concn. (μg mL−1) | 25 | 50 | 25 | 50 | 25 | 50 | 25 | 50 |
| Standard AB/AF | 26.33 ± 0.58 | 28.67 ± 1.15 | 25.67 ± 0.58 | 26.67 ± 0.58 | 20.00 ± 0.00 | 26.33 ± 0.58 | 17.00 ± 1.00 | 20.00 ± 0.00 |
| Control | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 00 |
| 6a | 7.33 ± 0.58 | 13.67 ± 0.58 | 6.67 ± 0.58 | 7.00 ± 0.00 | — | — | — | — |
| 6b | 8.33 ± 0.58 | 13.00 ± 1.00 | — | — | — | — | 7.00 ± 0.00 | 8.00 ± 0.00 |
| 6c | 8.33 ± 0.58 | 8.33 ± 0.58 | — | 7.00 ± 0.00 | 8.00 ± 0.00 | 10.00 ± 0.00 | — | — |
| 6d | — | 15.33 ± 0.58 | 9.33 ± 0.58 | 9.67 ± 1.15 | — | 7.83 ± 0.29 | — | — |
| 6e | 7.17 ± 0.29 | 9.33 ± 0.58 | 17.00 ± 0.00 | 18.33 ± 0.58 | 8.50 ± 0.50 | 10.00 ± 0.00 | — | — |
| 7a | — | 15.00 ± 0.00 | 8.00 ± 0.00 | 8.33 ± 0.58 | 8.33 ± 0.58 | 10.00 ± 0.00 | 7.17 ± 0.29 | 8.67 ± 0.58 |
| 7b | 8.33 ± 0.58 | 11.33 ± 0.58 | 11.00 ± 0.00 | 11.33 ± 0.58 | 8.00 ± 0.00 | 10.00 ± 0.00 | — | — |
| 7c | 10.67 ± 0.58 | 10.67 ± 0.58 | 6.33 ± 0.58 | 7.00 ± 0.00 | 7.83 ± 0.29 | 9.33 ± 0.58 | — | — |
| 7d | 7.00 ± 0.00 | 7.83 ± 0.29 | — | 6.83 ± 0.29 | — | — | 7.17 ± 0.29 | 8.00 ± 0.00 |
| 7e | 7.00 ± 0.00 | 10.67 ± 0.58 | — | — | — | — | 12.67 ± 0.58 | 12.67 ± 0.58 |
| 7f | 7.17 ± 0.29 | 14.67 ± 0.58 | — | 8.33 ± 0.58 | 7.17 ± 0.29 | 8.00 ± 0.00 | — | — |
| 7g | 7.83 ± 0.29 | 9.67 ± 0.58 | 13.67 ± 0.58 | 13.67 ± 0.58 | 7.33 ± 0.29 | 8.33 ± 0.29 | — | — |
| 9a | 8.00 ± 0.00 | 9.33 ± 0.58 | 9.33 ± 0.58 | 11.33 ± 0.58 | 9.00 ± 0.00 | 10.00 ± 0.00 | — | — |
| 9b | 10.67 ± 0.58 | 10.67 ± 0.58 | 7.00 ± 0.00 | 8.67 ± 0.58 | 9.33 ± 0.58 | 9.67 ± 0.58 | — | — |
| 9c | 12.67 ± 0.58 | 12.67 ± 0.58 | 10.00 ± 0.00 | 10.33 ± 0.58 | 9.33 ± 0.58 | 10.33 ± 0.58 | 12.00 ± 0.00 | 12.33 ± 0.58 |
| 9d | 7.00 ± 0.00 | 10.33 ± 0.58 | 10.67 ± 0.58 | 11.00 ± 0.00 | 8.00 ± 0.00 | 8.67 ± 0.29 | — | — |
| 9e | 8.67 ± 0.58 | 8.67 ± 0.58 | — | — | 7.67 ± 0.29 | 9.33 ± 0.58 | — | — |
| 9f | 8.67 ± 0.58 | 8.67 ± 0.29 | — | — | 8.00 ± 0.00 | 8.67 ± 0.58 | 8.33 ± 0.58 | 9.67 ± 0.58 |
| Compound | MIC in μg mL−1 | |||
|---|---|---|---|---|
| M. smegmatis | P. aeroginosa | S. aureus | C. albicans | |
| a AB; anti-bacterial standard ciprofloxacin; AF; anti-fungal standard fluconazole; —; not detected inhibition; control; dimethylsulfoxide. | ||||
| 6a | 25 | 25 | 50 | 50 |
| 6b | 25 | 50 | 50 | 25 |
| 6c | 12.5 | 12.5 | 25 | 12.5 |
| 6d | 6.25 | 12.5 | 25 | 12.5 |
| 6e | 6.25 | 12.5 | 25 | 12.5 |
| 7a | 12.5 | 12.5 | 25 | 12.5 |
| 7b | 12.5 | 12.5 | 25 | 12.5 |
| 7c | 25 | 12.5 | 50 | 25 |
| 7d | 12.5 | 25 | 50 | 25 |
| 7e | 25 | 50 | 50 | 12.5 |
| 7f | 12.5 | 12.5 | 50 | 25 |
| 7g | 25 | 25 | 50 | 12.5 |
| 9a | 12.5 | 12.5 | 50 | 25 |
| 9b | 12.5 | 12.5 | 50 | 25 |
| 9a | 12.5 | 12.5 | 50 | 25 |
| 9b | 25 | 25 | 25 | 25 |
| 9c | 12.5 | 12.5 | 50 | 25 |
| 9d | 25 | 25 | 25 | 25 |
| 9e | 25 | 25 | 50 | 25 |
| 9f | 12.5 | 12.5 | 50 | 25 |
| AB | <5 | <5 | <5 | — |
| AF | — | — | — | <10 |
2.3. Acute toxicity and gross behavioral studies
The acute oral toxicity study for the newly synthesized organic compounds 6a–e, 7a–g and 9a–f was carried out by the following OECD guidelines no. 420 (OECD Guidelines, 2008).34,35 Each group consisting of 6 mice (overnight fasted) and kept in colony cage at 25 ± 2 °C with 55% relative humidity and 12 hours of light and dark cycle. A specified dose of 100, 250, 500, 750, 1000, 1500 and 2000 mg kg−1 body weight of mice was administered orally as a single dose as a fine suspension prepared in saline using gum acacia powder. The acute toxic symptoms and the behavioral changes produced by the test compounds were observed continuously for 4 h periods at 8th, 12th and 24th h on set of toxic symptoms and the gross behavioral changes were also recorded. These animals were maintained for further 10 days with observation made daily.2.4. Biological results
The antimicrobial screening in well diffusion method revealed that, few of the tested compounds showed excellent inhibition against tested microbial strains. Among the synthesized compounds, 7a and 9c showed significant antimicrobial activity against all the tested microorganisms. The enhanced activity may be due to presence of trifluoromethyl functional group at seventh position of the quinoline core moiety and electron donating groups at third (7a: 4-methoxyphenyl) and fourth (9c: ethanolamine) position of quinoline ring. The compounds 6c, 6d, 6e, 7b, 7c, 7f, 9a, 9b and 9d are exhibited excellent antimicrobial activity against all the microorganisms except the filamentous fungi Penicillium chrysogenum. Among all the compounds, 6d is inhibiting Mycobacterium smegmatis to the maximum of 16 mm diameter may be due to the presence of strong electron withdrawing group (NO2) on pyrazole derivative at third position of 7-trifluoromethy-4-hydroxyquinoline. The presence of 1-phenyl-3-p-tolyl-1H-pyrazole carbohydrazide at third position of the 7-trifluoromethy-4-hydroxyquinoline may be the reason for the enhanced activity of 6e against Gram-negative bacteria Pseudomonas aeruginosa (maximum extent of 19 mm). Six of these compounds inhibited the filamentous fungi Penicillium chrysogenum at 25 μg mL−1 concentration. Except 6b, 7e, 9e and 9f, all compounds inhibited Gram-negative bacteria P. aeruginosa. MIC of 6d and 6e showed that are most active compounds against M. smigmatis with value of 6.25 μg mL−1 each. Nine compounds are having better MIC values (12.5 μg mL−1) for M. smigmatis compared to other microorganisms. All the compounds are showing lower activity against Gram positive bacteria S. aureus except few of the 6 series compounds having 25 μg mL−1 MIC. Results of antimicrobial studies have been presented in Tables 3 and 4.3. Conclusion
Two series of new 4-hydroxy-7-(trifluoromethyl)quinoline-3-carbohydrazide (6a–e and 7a–g) derivatives and N-alkyl-3-(5-phenyl-1,3,4-oxadiazol-2-yl)-7-(trifluoromethyl)quinolin-4-amine derivatives (9a–f) were synthesized in reasonably good yields. They were characterized by 1H NMR, 13C NMR, mass spectrometry, IR studies and elemental analyses. The structure of 9a has also been confirmed by X-ray crystallographic study. All the newly synthesized compounds were screened for in vitro antimicrobial activity by well plate method and MIC was determined using serial dilution method. Among the screened samples, 7a and 9c are showed significant antimicrobial activity against all the tested microbial strains in well diffusion method. Whereas, from the MIC studies 6d and 6e found to be the most active compounds among all others.The compound 9c has showed significant inhibition against all the tested micro organisms as compared to other synthesized compounds, which may be due to the presence of 7-trifluoromethy, 3-(5-phenyl-1,3,4-oxadiazol) and biologically active amines (ethanolamine) at fourth position of quinoline ring. Compound 7b has also shown good inhibition against both bacterial and fungal strains. This is possibly due to the presence of 3-(3,4-dimethoxy phenyl) carbohydrazide, 4-hydroxy and 7-trifluoromethyl groups on quinoline ring. The compounds containing pyrazole carbohydrazide derivatives and methoxyphenyl, N-diethylphenyl carbohydrazides at position 3 of 4-hydroxy-(7-trifluoromethy)quinoline accounted for the enhanced activity of Mycobacterium smegmatis up to 12–16 mm zone of inhibition of the compounds 6a, 6b, 6d, 7a, 7b, and 7f. In conclusion, antibacterial activity increases with increase of electron withdrawing group on pyrazole carbohydrazide and electron donating groups on phenylcarbohydrazide at third position of quinoline. In oxadiazole series (9a–f), antibacterial activity increases with introducing aliphatic amines at fourth position of the 7-(trifluoromethyl)-3-(5-phenyl-1,3,4-oxadiazol-2-yl)quinoline instead of aromatic amine.
As regards the relationship between the structure of the heterocyclic scaffold and the detected antimicrobial properties, it can be concluded that the combination of two different heterocyclic moieties namely quinoline and heterocyclic carbohydrazides, 1,3,4-oxadiazoles has enhanced biological activity and hence they are ideally suited for further notification to obtain more efficient antimicrobial compounds.
The acute oral toxicity study for the newly synthesized organic compounds 6a–e, 7a–g and 9a–f was performed and there were no mortality and significant behavioral changes observed for first 24 h for all newly synthesized compounds at all concentrations. But, the compounds 6b, 6d, 7a, 9b and 9f showed mortality at 750 mg kg−1 and above concentrations after 24 h. The remaining compounds are not showing any behavioral changes at all concentration throughout the experiment.
4. Experimental
4.1. Analysis and instruments
All the chemicals were purchased from Sigma Aldrich, Merck and S. D. Fine chemicals-India. Commercial grade solvents were used and were distilled before use. Melting points were determined by open capillary method and were uncorrected. The IR spectra (neat) were recorded on a JASCO FT/IR-4100 spectrophotometer and Bruker (400 MHz) spectrometer was used to record 1H-NMR and 13C-NMR spectra (DMSO-d6, CDCl3) using TMS as internal standard. Chemical shift values were given in δ (ppm) scales. The mass spectra were recorded on LC-MS-Agilent 1100 series and elemental analysis was performed on a Flash EA 1112 series CHNS-O Analyzer. The completion of the reactions was checked by thin layer chromatography (TLC) on silica gel coated aluminium sheets (silica gel 60 F254). The names of the structures were given as per chemdraw.4.2. Synthesis of diethyl ({[3-(trifluoromethyl)phenyl]amino}methylidene)propanedioate (3)
3-(Trifluoromethyl) aniline 1 (10.0 g, 0.062 mol) and diethyl ethoxymethylene malonate 2 (18.61 mL, 0.093 mol) were heated to 110 °C for 6 h. The reaction mixture was cooled to room temperature, the solid thus formed was taken in pet ether and stirred for 20 min and filtered to get compound 3 as a white crystalline solid. Yield: 19.0 g, 92%; m.p: 44–46 °C; IR (neat, νmax cm−1): 3252 (N–H), 3118, 2979 (C–H-str), 1708 and 1616 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (400 MHz, CDCl3): δ ppm 1.35 (t, −CH3, 3H, J = 5.3 Hz), 1.38 (t, –CH3, 3H, J = 5.3 Hz), 4.25 (q, –CH2, 2H), 4.34 (q, –CH2, 2H), 7.25 (m, –CH, 1H), 7.36 (d, –CH, 1H, J = 6.1 Hz), 7.62 (m, –CH, 2H), 8.45 (d, –NCH
O); 1H NMR (400 MHz, CDCl3): δ ppm 1.35 (t, −CH3, 3H, J = 5.3 Hz), 1.38 (t, –CH3, 3H, J = 5.3 Hz), 4.25 (q, –CH2, 2H), 4.34 (q, –CH2, 2H), 7.25 (m, –CH, 1H), 7.36 (d, –CH, 1H, J = 6.1 Hz), 7.62 (m, –CH, 2H), 8.45 (d, –NCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–, 1H, J = 9.6 Hz), 11.45 (brd, –NH, 1H, J = 13.8). 13C NMR (100 MHz, CDCl3): δ ppm 14.97, 59.61, 111.32, 119.81, 119.87, 122.61, 123.10, 130.92, 131.55; Anal. calcd. For C15H16F3NO4; calcd: C, 54.38; H, 4.80; N, 4.23; found: C, 54.35; H, 4.80; N, 4.20%.16
C–, 1H, J = 9.6 Hz), 11.45 (brd, –NH, 1H, J = 13.8). 13C NMR (100 MHz, CDCl3): δ ppm 14.97, 59.61, 111.32, 119.81, 119.87, 122.61, 123.10, 130.92, 131.55; Anal. calcd. For C15H16F3NO4; calcd: C, 54.38; H, 4.80; N, 4.23; found: C, 54.35; H, 4.80; N, 4.20%.16
4.3. Synthesis of 4-hydroxy-7-trifluoromethyl-quinoline-3-carboxylic acid ethyl ester (4)
Diethyl ({[3-(trifluoromethyl)phenyl]amino}methylidene)propanedioate 3 (10.0 g, 0.030 mol) and Dowtherm (100 mL) were heated to 250 °C for 5 h. The reaction mixture was then cooled to 25 °C and stirred in 150 mL hexane for 10 min. The solid product obtained was filtered and dried. The crude product obtained was purified by column chromatography using pet ether and ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as the eluent. Yield: 7.2 g, 84%; m.p: 298–300 °C; IR (neat, νmax cm−1): 3322 (–OH), 3029, 2970 (C–H-str), 1706 (C
5) as the eluent. Yield: 7.2 g, 84%; m.p: 298–300 °C; IR (neat, νmax cm−1): 3322 (–OH), 3029, 2970 (C–H-str), 1706 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (400 MHz, DMSO-d6): δ ppm 1.23 (t, 3H, J = 8.0 Hz, –CH3), 4.18 (q, 2H, –CH2), 7.53 (t, 1H, ArH, J = 8.0 Hz), 8.07 (d, 1H, ArH, 7.8 Hz), 8.41 (d, 2H, ArH, J = 8.0 Hz), 11.62 (s, 1H, –OH). 13C NMR (100 MHz, DMSO-d6): δ ppm 14.71, 60.49, 111.38, 119.10, 124.61, 125.35, 130.99, 131.55, 146.43. MS: m/z = 286 (M + 1); Anal. calcd. For C13H10F3NO3; calcd: C, 54.74; H, 3.53; N, 4.91; found: C, 54.77; H, 3.50; N, 4.95%.16
O); 1H NMR (400 MHz, DMSO-d6): δ ppm 1.23 (t, 3H, J = 8.0 Hz, –CH3), 4.18 (q, 2H, –CH2), 7.53 (t, 1H, ArH, J = 8.0 Hz), 8.07 (d, 1H, ArH, 7.8 Hz), 8.41 (d, 2H, ArH, J = 8.0 Hz), 11.62 (s, 1H, –OH). 13C NMR (100 MHz, DMSO-d6): δ ppm 14.71, 60.49, 111.38, 119.10, 124.61, 125.35, 130.99, 131.55, 146.43. MS: m/z = 286 (M + 1); Anal. calcd. For C13H10F3NO3; calcd: C, 54.74; H, 3.53; N, 4.91; found: C, 54.77; H, 3.50; N, 4.95%.16
4.4. Synthesis of 4-hydroxy-7-trifluoromethyl-quinoline-3-carboxylic acid hydrazide (5)
A mixture of ethyl 4-hydroxy-7-trifluoromethyl-quinoline-3-carboxylic acid ethyl ester 4 (5.0 g, 0.017 mol) and hydrazine hydrate (4.1 mL, 0.085 mol) in ethanol (50 mL) were refluxed for 4 h. After the completion of the reaction, the reaction mixture was concentrated and allowed to cool. The solid product obtained was filtered, washed with water and recrystallized from ethanol to give 5 as a white solid. Yield: 4.25 g, 89%; m.p: 255–257 °C; IR (neat, νmax cm−1): 3442 (–OH), 3296 and 3244 (N–H), 3088, 2963 (C–H-str), 1649 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (400 MHz, DMSO-d6): δ ppm 4.67 (s, 2H, –NH2), 7.76 (dd, 1H, ArH, J = 7.7 Hz, J = 1.4 Hz), 8.10 (s, 1H, ArH), 8.45 (d, 1H, ArH, J = 8.4 Hz), 8.90 (s, 1H, ArH), 10.57 (s, 1H, –NH), 12.88 (s, 1H, –OH); 13C NMR (100 MHz, DMSO-d6): δ ppm 110.68, 123.20, 126.53, 130.06, 131.25, 146.79, 160.96, 174.49; MS: m/z = 272 (M + 1). Anal. calcd. For C11H8F3N3O2; calcd: C, 48.72; H, 2.97; N, 15.49; found: C, 48.75; H, 2.97; N, 15.59%.28
O); 1H NMR (400 MHz, DMSO-d6): δ ppm 4.67 (s, 2H, –NH2), 7.76 (dd, 1H, ArH, J = 7.7 Hz, J = 1.4 Hz), 8.10 (s, 1H, ArH), 8.45 (d, 1H, ArH, J = 8.4 Hz), 8.90 (s, 1H, ArH), 10.57 (s, 1H, –NH), 12.88 (s, 1H, –OH); 13C NMR (100 MHz, DMSO-d6): δ ppm 110.68, 123.20, 126.53, 130.06, 131.25, 146.79, 160.96, 174.49; MS: m/z = 272 (M + 1). Anal. calcd. For C11H8F3N3O2; calcd: C, 48.72; H, 2.97; N, 15.49; found: C, 48.75; H, 2.97; N, 15.59%.28
4.5. General method for the preparation of 4-hydroxy-7-(trifluoromethyl) quinoline-3-carbohydrazide derivatives (6a–e and 7a–g)
An equimolar mixture of 4-hydroxy-7-trifluoromethyl-quinoline-3-carboxylic acid hydrazide 5 (0.5 g, 0.0018 mol), pyrazole-4-carbaldehyde or aromatic aldehydes (0.002 mol) and catalytic amount of acetic acid in dry ethanol (5 mL) were stirred at 25 °C for 1 h. Completion of the reaction was monitored by TLC. The precipitated solid was filtered under suction, washed with ethanol and recrystallized from ethanol.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1604 (C
O), 1604 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6): δ ppm 7.15–7.50 (m, 6H, ArH), 7.52 (d, 1H, ArH, J = 8.4 Hz), 7.75 (d, 2H, ArH, J = 8.0 Hz), 7.96 (d, 2H, ArH, J = 8.0 Hz), 8.07 (s, 1H, N
N); 1H NMR (400 MHz, DMSO-d6): δ ppm 7.15–7.50 (m, 6H, ArH), 7.52 (d, 1H, ArH, J = 8.4 Hz), 7.75 (d, 2H, ArH, J = 8.0 Hz), 7.96 (d, 2H, ArH, J = 8.0 Hz), 8.07 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.42 (d, 1H, ArH, J = 8.4 Hz), 8.43 (s, 1H, ArH), 8.96 (s, 2H, ArH), 12.90 (s, 1H, –OH), 13.10 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 10.51, 117.29, 117.77, 120.32, 128.00, 128.73, 128.91, 130.06, 138.11, 139.38, 139.23, 146.15, 155.07, 152.01, 160.55, 173.91; MS: m/z = 502 (M + 1). Anal. calcd. For C27H18F3N5O2; calcd: C, 64.67; H, 3.62; N, 13.97; found: C, 64.65; H, 3.63; N, 13.90%.
CH), 8.42 (d, 1H, ArH, J = 8.4 Hz), 8.43 (s, 1H, ArH), 8.96 (s, 2H, ArH), 12.90 (s, 1H, –OH), 13.10 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 10.51, 117.29, 117.77, 120.32, 128.00, 128.73, 128.91, 130.06, 138.11, 139.38, 139.23, 146.15, 155.07, 152.01, 160.55, 173.91; MS: m/z = 502 (M + 1). Anal. calcd. For C27H18F3N5O2; calcd: C, 64.67; H, 3.62; N, 13.97; found: C, 64.65; H, 3.63; N, 13.90%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1607 (C
O), 1607 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1176 (O–CH3); 1H NMR (400 MHz, DMSO-d6): δ ppm 3.86 (s, 3H, OCH3), 7.10 (d, 2H, ArH, J = 8.6 Hz), 7.38 (t, 1H, ArH, J = 7.4 Hz), 7.55 (t, 2H, ArH, J = 8.1 Hz), 7.76 (d, 2H, ArH, J = 8.6 Hz), 7.82 (d, 1H, ArH, J = 9.0 Hz), 8.02 (d, 2H, ArH, J = 7.7 Hz), 8.13 (s, 1H, N
N), 1176 (O–CH3); 1H NMR (400 MHz, DMSO-d6): δ ppm 3.86 (s, 3H, OCH3), 7.10 (d, 2H, ArH, J = 8.6 Hz), 7.38 (t, 1H, ArH, J = 7.4 Hz), 7.55 (t, 2H, ArH, J = 8.1 Hz), 7.76 (d, 2H, ArH, J = 8.6 Hz), 7.82 (d, 1H, ArH, J = 9.0 Hz), 8.02 (d, 2H, ArH, J = 7.7 Hz), 8.13 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.45 (s, 1H, ArH), 8.48 (d, 1H, ArH, J = 8.6 Hz), 8.98 (s, 1H, ArH), 9.03 (s, 1H, ArH), 12.99 (s, 1H, –OH), 13.15 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 55.62, 111.43, 118.72, 119.23, 121.28, 124.67, 124.90, 126.69, 127.32, 127.67, 127.88, 129.74, 130.07, 138.96, 146.26, 152.23, 159.54, 175.85. MS: m/z = 532 (M + 1). Anal. calcd. For C28H20F3N5O3; calcd: C, 63.28; H, 3.79; N, 13.18; found: C, 63.34; H, 3.72; N, 13.20%.
CH), 8.45 (s, 1H, ArH), 8.48 (d, 1H, ArH, J = 8.6 Hz), 8.98 (s, 1H, ArH), 9.03 (s, 1H, ArH), 12.99 (s, 1H, –OH), 13.15 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 55.62, 111.43, 118.72, 119.23, 121.28, 124.67, 124.90, 126.69, 127.32, 127.67, 127.88, 129.74, 130.07, 138.96, 146.26, 152.23, 159.54, 175.85. MS: m/z = 532 (M + 1). Anal. calcd. For C28H20F3N5O3; calcd: C, 63.28; H, 3.79; N, 13.18; found: C, 63.34; H, 3.72; N, 13.20%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1605 (C
O), 1605 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6): δ ppm 7.34–7.55 (m, 4H, ArH), 7.75 (d, 2H, ArH, J = 8.0 Hz), 7.83 (d, 2H, ArH, J = 8.0 Hz), 7.96–7.98 (m, 3H, ArH), 8.06 (s, 1H, N
N); 1H NMR (400 MHz, DMSO-d6): δ ppm 7.34–7.55 (m, 4H, ArH), 7.75 (d, 2H, ArH, J = 8.0 Hz), 7.83 (d, 2H, ArH, J = 8.0 Hz), 7.96–7.98 (m, 3H, ArH), 8.06 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.42 (s, 1H, ArH), 8.95 (s, 1H, ArH), 8.97 (s, 1H, ArH), 12.96 (s, 1H, –OH), 13.10 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 111.02, 117.49, 117.82, 120.45, 129.02, 129.14, 130.23, 131.15, 138.33, 139.54, 139.77, 146.48, 156.00, 153.15, 160.78, 175.02; MS: m/z = 536 (M + 1). Anal. calcd. For C27H17ClF3N5O2; calcd: C, 60.51; H, 3.20; N, 13.07; found: C, 60.58; H, 3.21; N, 13.05%.
CH), 8.42 (s, 1H, ArH), 8.95 (s, 1H, ArH), 8.97 (s, 1H, ArH), 12.96 (s, 1H, –OH), 13.10 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 111.02, 117.49, 117.82, 120.45, 129.02, 129.14, 130.23, 131.15, 138.33, 139.54, 139.77, 146.48, 156.00, 153.15, 160.78, 175.02; MS: m/z = 536 (M + 1). Anal. calcd. For C27H17ClF3N5O2; calcd: C, 60.51; H, 3.20; N, 13.07; found: C, 60.58; H, 3.21; N, 13.05%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1596 (C
O), 1596 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1534 (N–O), 1495 (N–O); 1H NMR (400 MHz, DMSO-d6): δ ppm 7.41–7.45 (m, 1H, ArH), 7.56–7.60 (m, 2H, ArH), 7.83 (dd, 1H, ArH, J = 8.76 Hz, J = 1.56 Hz), 8.04–8.07 (m, 2H, ArH), 8.14 (s, 1H, N
N), 1534 (N–O), 1495 (N–O); 1H NMR (400 MHz, DMSO-d6): δ ppm 7.41–7.45 (m, 1H, ArH), 7.56–7.60 (m, 2H, ArH), 7.83 (dd, 1H, ArH, J = 8.76 Hz, J = 1.56 Hz), 8.04–8.07 (m, 2H, ArH), 8.14 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.23–8.26 (m, 2H, ArH), 8.36–8.39 (m, 2H, ArH), 8.49 (d, 1H, ArH, J = 8.4 Hz), 8.56 (s, 1H, ArH), 9.0 (s, 2H, ArH), 13.03 (s, 1H, –OH), 13.14 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 111.81, 117.72, 117.81, 120.63, 129.84, 129.97, 131.04, 131.26, 138.59, 139.87, 139.94, 147.00, 156.12, 153.25, 161.02, 175.49; MS: m/z = 547 (M + 1). Anal. calcd. For C27H17F3N6O4; calcd: C, 59.34; H, 3.14; N, 15.38; found: C, 59.38; H, 3.11; N, 15.40%.
CH), 8.23–8.26 (m, 2H, ArH), 8.36–8.39 (m, 2H, ArH), 8.49 (d, 1H, ArH, J = 8.4 Hz), 8.56 (s, 1H, ArH), 9.0 (s, 2H, ArH), 13.03 (s, 1H, –OH), 13.14 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 111.81, 117.72, 117.81, 120.63, 129.84, 129.97, 131.04, 131.26, 138.59, 139.87, 139.94, 147.00, 156.12, 153.25, 161.02, 175.49; MS: m/z = 547 (M + 1). Anal. calcd. For C27H17F3N6O4; calcd: C, 59.34; H, 3.14; N, 15.38; found: C, 59.38; H, 3.11; N, 15.40%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1605 (C
O), 1605 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6): δ ppm 2.41(s, 3H, CH3), 7.36–7.40 (m, 3H, ArH), 7.55 (t, 2H, ArH, J = 8.0 Hz), 7.70 (d, 2H, ArH, J = 7.8 Hz), 7.83 (d, 1H, ArH, J = 8.7 Hz), 8.01 (d, 2H, ArH, J = 8.0 Hz), 8.13 (s, 1H, N
N); 1H NMR (400 MHz, DMSO-d6): δ ppm 2.41(s, 3H, CH3), 7.36–7.40 (m, 3H, ArH), 7.55 (t, 2H, ArH, J = 8.0 Hz), 7.70 (d, 2H, ArH, J = 7.8 Hz), 7.83 (d, 1H, ArH, J = 8.7 Hz), 8.01 (d, 2H, ArH, J = 8.0 Hz), 8.13 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.46 (s, 1H, ArH), 8.47 (d, 1H, ArH, J = 8.7 Hz), 8.98 (s, 1H, ArH), 9.02 (s, 1H, ArH), 12.96 (s, 1H, –OH), 13.13 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 21.18, 111.68, 117.25, 117.42, 121.32, 127.88, 128.83, 129.80, 130.06, 138.51, 139.12, 139.38, 139.56, 141.27, 146.11, 152.07, 160.96, 175.49. MS: m/z = 516 (M + 1). Anal. calcd. For C27H17F3N6O4; calcd: C, 65.24; H, 3.91; N, 13.59; found: C, 65.30; H, 3.89; N, 13.55%.
CH), 8.46 (s, 1H, ArH), 8.47 (d, 1H, ArH, J = 8.7 Hz), 8.98 (s, 1H, ArH), 9.02 (s, 1H, ArH), 12.96 (s, 1H, –OH), 13.13 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 21.18, 111.68, 117.25, 117.42, 121.32, 127.88, 128.83, 129.80, 130.06, 138.51, 139.12, 139.38, 139.56, 141.27, 146.11, 152.07, 160.96, 175.49. MS: m/z = 516 (M + 1). Anal. calcd. For C27H17F3N6O4; calcd: C, 65.24; H, 3.91; N, 13.59; found: C, 65.30; H, 3.89; N, 13.55%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1605 (C
O), 1605 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1165 (O–CH3); 1H NMR (400 MHz, CDCl3): δ ppm 2.95 (s, 3H, OCH3), 6.17 (d, 2H, ArH, J = 8.2 Hz), 6.86 (d, 2H, ArH, J = 8.2 Hz), 6.96 (d, 2H, ArH, J = 8.0 Hz), 7.28 (s, 1H, N
N), 1165 (O–CH3); 1H NMR (400 MHz, CDCl3): δ ppm 2.95 (s, 3H, OCH3), 6.17 (d, 2H, ArH, J = 8.2 Hz), 6.86 (d, 2H, ArH, J = 8.2 Hz), 6.96 (d, 2H, ArH, J = 8.0 Hz), 7.28 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.53 (s, 1H, ArH), 7.63 (d, 1H, ArH, J = 8.0 Hz), 8.15 (s, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ ppm 55.13, 108.83, 112.10, 117.32, 121.34, 122.01, 127.33, 127.85, 128.61, 139.41, 146.05, 148.55, 149.52, 151.38, 160.98, 160.99, 175.87; MS: m/z = 390 (M + 1). Anal. calcd. For C19H14F3N3O3; calcd: C, 58.61; H, 3.62; N, 10.79; found: C, 58.72; H, 3.62; N, 10.78%.
CH), 7.53 (s, 1H, ArH), 7.63 (d, 1H, ArH, J = 8.0 Hz), 8.15 (s, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ ppm 55.13, 108.83, 112.10, 117.32, 121.34, 122.01, 127.33, 127.85, 128.61, 139.41, 146.05, 148.55, 149.52, 151.38, 160.98, 160.99, 175.87; MS: m/z = 390 (M + 1). Anal. calcd. For C19H14F3N3O3; calcd: C, 58.61; H, 3.62; N, 10.79; found: C, 58.72; H, 3.62; N, 10.78%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1582 (C
O), 1582 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1173 (O–CH3), 1156 (O–CH3); 1H NMR (400 MHz, DMSO-d6): δ ppm 3.82 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 7.06 (d, 1H, ArH, J = 4.4 Hz), 7.28 (dd, 1H, ArH, J = 8.3 Hz, J = 1.8 Hz), 7.39 (d, 1H, ArH, J = 1.8 Hz), 7.82 (dd, 1H, ArH, J = 8.6 Hz, J = 1.3 Hz), 8.15 (s, 1H, N
N), 1173 (O–CH3), 1156 (O–CH3); 1H NMR (400 MHz, DMSO-d6): δ ppm 3.82 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 7.06 (d, 1H, ArH, J = 4.4 Hz), 7.28 (dd, 1H, ArH, J = 8.3 Hz, J = 1.8 Hz), 7.39 (d, 1H, ArH, J = 1.8 Hz), 7.82 (dd, 1H, ArH, J = 8.6 Hz, J = 1.3 Hz), 8.15 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.38 (s, 1H, ArH), 8.50 (d, 1H, ArH, J = 8.4 Hz), 9.02 (s, 1H, ArH), 13.02 (s, 1H, –OH), 13.11 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 55.97, 108.80, 112.09, 117.39, 121.35, 122.06, 127.33, 127.93, 128.53, 139.33, 146.04, 148.60, 149.51, 151.28, 160.97, 160.97, 175.74. MS: m/z = 420 (M + 1). Anal. calcd. For C20H16F3N3O4; calcd: C, 57.28; H, 3.85; N, 10.02; found: C, 57.35; H, 3.85; N, 10.00%.
CH), 8.38 (s, 1H, ArH), 8.50 (d, 1H, ArH, J = 8.4 Hz), 9.02 (s, 1H, ArH), 13.02 (s, 1H, –OH), 13.11 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 55.97, 108.80, 112.09, 117.39, 121.35, 122.06, 127.33, 127.93, 128.53, 139.33, 146.04, 148.60, 149.51, 151.28, 160.97, 160.97, 175.74. MS: m/z = 420 (M + 1). Anal. calcd. For C20H16F3N3O4; calcd: C, 57.28; H, 3.85; N, 10.02; found: C, 57.35; H, 3.85; N, 10.00%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1607 (C
O), 1607 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6): δ ppm 7.15–7.18 (m, 1H, ArH), 7.47 (d, 1H, ArH, J = 7.6 Hz), 7.69–7.70 (m, 1H, ArH), 7.83 (d, 1H, ArH, J = 8.5 Hz), 8.15 (s, 1H, N
N); 1H NMR (400 MHz, DMSO-d6): δ ppm 7.15–7.18 (m, 1H, ArH), 7.47 (d, 1H, ArH, J = 7.6 Hz), 7.69–7.70 (m, 1H, ArH), 7.83 (d, 1H, ArH, J = 8.5 Hz), 8.15 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.49 (d, 1H, ArH, J = 8.4 Hz), 8.71 (s, 1H, ArH), 9.01 (s, 1H, ArH), 13.03 (s, 1H, –OH), 13.12 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 120.31, 122.54, 122.81, 123.10, 127.13, 127.74, 146.29, 149.15, 151.06, 160.27, 161.92, 174.35; MS: m/z = 366 (M + 1). Anal. calcd. For C16H10F3N3O2S; calcd: C, 52.60; H, 2.76; N, 11.50; found: C, 52.66; H, 2.72; N, 11.53%.
CH), 8.49 (d, 1H, ArH, J = 8.4 Hz), 8.71 (s, 1H, ArH), 9.01 (s, 1H, ArH), 13.03 (s, 1H, –OH), 13.12 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 120.31, 122.54, 122.81, 123.10, 127.13, 127.74, 146.29, 149.15, 151.06, 160.27, 161.92, 174.35; MS: m/z = 366 (M + 1). Anal. calcd. For C16H10F3N3O2S; calcd: C, 52.60; H, 2.76; N, 11.50; found: C, 52.66; H, 2.72; N, 11.53%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1602 (C
O), 1602 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6): δ ppm 2.97 (s, 6H, NCH3), 6.77 (d, 2H, ArH, J = 8.9 Hz), 7.58 (d, 1H, ArH, J = 8.9 Hz), 7.76 (d, 1H, ArH, J = 8.4 Hz), 8.12 (s, 1H, N
N); 1H NMR (400 MHz, DMSO-d6): δ ppm 2.97 (s, 6H, NCH3), 6.77 (d, 2H, ArH, J = 8.9 Hz), 7.58 (d, 1H, ArH, J = 8.9 Hz), 7.76 (d, 1H, ArH, J = 8.4 Hz), 8.12 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.26 (s, 1H, ArH), 8.48 (d, 1H, ArH, J = 8.4 Hz), 9.00 (s, 1H, ArH), 13.11 (s, 1H, –OH), 13.14 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 47.05, 53.04, 111.73, 112.04, 117.72, 121.36, 122.38, 127.02, 129.09, 138.96, 145.97, 148.92, 151.27, 160.14, 175.51. MS: m/z = 403 (M + 1). Anal. calcd. For C20H17F3N4O2; calcd: C, 59.70; H, 4.26; N, 13.92; found: C, 59.68; H, 4.25; N, 13.82%.
CH), 8.26 (s, 1H, ArH), 8.48 (d, 1H, ArH, J = 8.4 Hz), 9.00 (s, 1H, ArH), 13.11 (s, 1H, –OH), 13.14 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 47.05, 53.04, 111.73, 112.04, 117.72, 121.36, 122.38, 127.02, 129.09, 138.96, 145.97, 148.92, 151.27, 160.14, 175.51. MS: m/z = 403 (M + 1). Anal. calcd. For C20H17F3N4O2; calcd: C, 59.70; H, 4.26; N, 13.92; found: C, 59.68; H, 4.25; N, 13.82%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1615 (C
O), 1615 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1206 (O–CH3); 1H NMR (400 MHz, DMSO-d6): δ ppm 1.37 (t, 3H, CH3, J = 6.8 Hz), 4.07 (q, 2H, OCH2, J = 4.4 Hz), 6.87 (t, 1H, ArH, J = 8.4 Hz), 7.03 (d, 1H, ArH, J = 7.7 Hz), 7.12 (d, 1H, ArH, J = 7.0 Hz), 7.84 (d, 1H, ArH, J = 7.1 Hz), 8.15 (s, 1H, N
N), 1206 (O–CH3); 1H NMR (400 MHz, DMSO-d6): δ ppm 1.37 (t, 3H, CH3, J = 6.8 Hz), 4.07 (q, 2H, OCH2, J = 4.4 Hz), 6.87 (t, 1H, ArH, J = 8.4 Hz), 7.03 (d, 1H, ArH, J = 7.7 Hz), 7.12 (d, 1H, ArH, J = 7.0 Hz), 7.84 (d, 1H, ArH, J = 7.1 Hz), 8.15 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.50 (d, 1H, ArH, J = 7.0 Hz), 8.69 (s, 1H, ArH), 9.04 (s, 1H, ArH), 11.14 (s, 1H, –OH), 13.13 (s, 1H, –OH), 13.18 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 21.40, 64.52, 111.84, 117.13, 121.98, 122.45, 123.08, 126.33, 127.81, 128.05, 140.00, 145.99, 150.21, 159.27, 160.87, 160.97, 176.02; MS: m/z = 420 (M + 1). Anal. calcd. For C20H16F3N3O4; calcd: C, 57.28; H, 3.85; N, 10.02; found: C, 57.30; H, 3.86; N, 10.00%.
CH), 8.50 (d, 1H, ArH, J = 7.0 Hz), 8.69 (s, 1H, ArH), 9.04 (s, 1H, ArH), 11.14 (s, 1H, –OH), 13.13 (s, 1H, –OH), 13.18 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 21.40, 64.52, 111.84, 117.13, 121.98, 122.45, 123.08, 126.33, 127.81, 128.05, 140.00, 145.99, 150.21, 159.27, 160.87, 160.97, 176.02; MS: m/z = 420 (M + 1). Anal. calcd. For C20H16F3N3O4; calcd: C, 57.28; H, 3.85; N, 10.02; found: C, 57.30; H, 3.86; N, 10.00%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1586 (C
O), 1586 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6): δ ppm 1.12 (t, 6H, CH3, J = 6.9 Hz), 3.37 (q, 4H, NCH2, J = 7.0 Hz), 6.13 (s, 1H, ArH), 6.29 (dd, 1H, ArH, J = 8.8 Hz, J = 2.4 Hz), 7.22 (d, 1H, ArH, J = 8.8 Hz), 7 0.83 (dd, 1H, ArH, J = 8.6 Hz, J = 1.4 Hz), 8.14 (s, 1H, N
N); 1H NMR (400 MHz, DMSO-d6): δ ppm 1.12 (t, 6H, CH3, J = 6.9 Hz), 3.37 (q, 4H, NCH2, J = 7.0 Hz), 6.13 (s, 1H, ArH), 6.29 (dd, 1H, ArH, J = 8.8 Hz, J = 2.4 Hz), 7.22 (d, 1H, ArH, J = 8.8 Hz), 7 0.83 (dd, 1H, ArH, J = 8.6 Hz, J = 1.4 Hz), 8.14 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.46 (s, 1H, ArH), 8.49 (d, 1H, ArH, J = 8.4 Hz), 9.00 (s, 1H, ArH), 11.37 (s, 1H, –OH), 12.91 (s, 1H, –OH), 13.10 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 17.54, 54.01, 111.66, 117.62, 121.87, 122.50, 123.16, 126.28, 127.15, 128.10, 145.83, 150.12, 159.30, 160.88, 161.00, 175.95; MS: m/z = 447 (M + 1). Anal. calcd. For C22H21F3N4O3; calcd: C, 59.19; H, 4.74; N, 12.55; found: C, 59.20; H, 4.72; N, 12.15%.
CH), 8.46 (s, 1H, ArH), 8.49 (d, 1H, ArH, J = 8.4 Hz), 9.00 (s, 1H, ArH), 11.37 (s, 1H, –OH), 12.91 (s, 1H, –OH), 13.10 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 17.54, 54.01, 111.66, 117.62, 121.87, 122.50, 123.16, 126.28, 127.15, 128.10, 145.83, 150.12, 159.30, 160.88, 161.00, 175.95; MS: m/z = 447 (M + 1). Anal. calcd. For C22H21F3N4O3; calcd: C, 59.19; H, 4.74; N, 12.55; found: C, 59.20; H, 4.72; N, 12.15%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1580 (C
O), 1580 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6): δ ppm 7.84 (dd, 1H, ArH, J = 8.6 Hz, J = 1.5 Hz), 8.18 (s, 1H, N
N); 1H NMR (400 MHz, DMSO-d6): δ ppm 7.84 (dd, 1H, ArH, J = 8.6 Hz, J = 1.5 Hz), 8.18 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.34–8.35 (m, 1H, ArH), 8.52 (s, 1H, ArH), 8.76–8.78 (m, 2H, ArH), 8.89 (d, 1H, ArH, J = 8.2 Hz), 9.06 (s, 1H, ArH), 13.16 (s, 1H, –OH), 13.28 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 117.98, 119.26, 120.99, 121.16, 122.72, 123.15, 126.32, 127.31, 145.89, 150.15, 159.39, 160.87, 161.03, 175.72; MS: m/z = 440 (M + 1). Anal. calcd. For C17H10BrF3N4O2; calcd: C, 46.49; H, 2.30; N, 12.76; found: C, 46.45; H, 2.32; N, 12.71%.
CH), 8.34–8.35 (m, 1H, ArH), 8.52 (s, 1H, ArH), 8.76–8.78 (m, 2H, ArH), 8.89 (d, 1H, ArH, J = 8.2 Hz), 9.06 (s, 1H, ArH), 13.16 (s, 1H, –OH), 13.28 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6): δ ppm 117.98, 119.26, 120.99, 121.16, 122.72, 123.15, 126.32, 127.31, 145.89, 150.15, 159.39, 160.87, 161.03, 175.72; MS: m/z = 440 (M + 1). Anal. calcd. For C17H10BrF3N4O2; calcd: C, 46.49; H, 2.30; N, 12.76; found: C, 46.45; H, 2.32; N, 12.71%.4.6. Synthesis of 4-chloro-3-(5-phenyl-1,3,4-oxadiazol-2-yl)-7-(trifluoromethyl)quinoline (8)
A mixture of 4-hydroxy-7-(trifluoromethyl)quinoline-3-carbohydrazide 5 (5.0 g, 0.018 mol), benzoic acid (2.44 g, 0.020 mol) and phosphorous oxychloride (50 mL) were heated at 100 °C for 10 h. The reaction mixture was then cooled to room temperature, the excess of POCl3 was removed by distillation under vacuum. The residue obtained was quenched to crushed ice and the solid separated was filtered off and dried through pump. The crude product was purified by column chromatography using pet ether and ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent.
1) as the eluent.
Yield: 3.1 g, 45%; m.p: 131–133 °C; IR (neat, νmax cm−1): 3049, 2921 (C–H-str), 1596 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6): δ ppm 7.58–7.64 (m, 2H, ArH), 8.10 (t, 2H, ArH, J = 8.0 Hz), 8.15 (d, 2H, ArH, J = 8.0 Hz), 8.53 (s, 1H, ArH), 8.62 (d, 1H, ArH, J = 8.0 Hz), 9.62 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 123.35, 125.03, 127.17, 127.17, 127.76, 127.92, 129.03, 129.73, 129.91, 130.06, 131.22, 132.54, 133.32, 151.48, 165.30, 167.78; MS: m/z = 376 (M + 1). Anal. calcd. For C18H9ClF3N3O; calcd: C, 57.54; H, 2.41; N, 11.18; found: C, 57.55; H, 2.38; N, 11.20%.
N); 1H NMR (400 MHz, DMSO-d6): δ ppm 7.58–7.64 (m, 2H, ArH), 8.10 (t, 2H, ArH, J = 8.0 Hz), 8.15 (d, 2H, ArH, J = 8.0 Hz), 8.53 (s, 1H, ArH), 8.62 (d, 1H, ArH, J = 8.0 Hz), 9.62 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 123.35, 125.03, 127.17, 127.17, 127.76, 127.92, 129.03, 129.73, 129.91, 130.06, 131.22, 132.54, 133.32, 151.48, 165.30, 167.78; MS: m/z = 376 (M + 1). Anal. calcd. For C18H9ClF3N3O; calcd: C, 57.54; H, 2.41; N, 11.18; found: C, 57.55; H, 2.38; N, 11.20%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6): δ ppm 2.26 (s, 3H, NCH3), 2.57 (t, 4H, NCH2, J = 4.2 Hz), 3.21 (t, 4H, NCH2, J = 4.6 Hz), 7.67–7.70 (m, 3H, ArH), 7.97 (dd, 1H, ArH, J = 8.8 Hz, J = 1.84 Hz), 8.12–8.15 (m, 2H, ArH), 8.39 (s, 1H, ArH), 8.43 (d, 1H, ArH, J = 8.8 Hz), 9.08 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 46.29, 51.91, 55.22, 55.59, 111.59, 115.86, 122.72, 123.68, 127.14, 127.23, 127.33, 127.50, 127.66, 130.04, 149.36, 151.50, 153.56, 155.80, 162.37, 165.28. MS: m/z = 440 (M + 1). Anal. calcd. For C23H20F3N5O; calcd: C, 62.86; H, 4.59; N, 15.94; found: C, 62.96; H, 4.55; N, 15.98%.
N); 1H NMR (400 MHz, DMSO-d6): δ ppm 2.26 (s, 3H, NCH3), 2.57 (t, 4H, NCH2, J = 4.2 Hz), 3.21 (t, 4H, NCH2, J = 4.6 Hz), 7.67–7.70 (m, 3H, ArH), 7.97 (dd, 1H, ArH, J = 8.8 Hz, J = 1.84 Hz), 8.12–8.15 (m, 2H, ArH), 8.39 (s, 1H, ArH), 8.43 (d, 1H, ArH, J = 8.8 Hz), 9.08 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 46.29, 51.91, 55.22, 55.59, 111.59, 115.86, 122.72, 123.68, 127.14, 127.23, 127.33, 127.50, 127.66, 130.04, 149.36, 151.50, 153.56, 155.80, 162.37, 165.28. MS: m/z = 440 (M + 1). Anal. calcd. For C23H20F3N5O; calcd: C, 62.86; H, 4.59; N, 15.94; found: C, 62.96; H, 4.55; N, 15.98%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6): δ ppm 3.15 (t, 4H, NCH2, J = 4.2 Hz), 4.92 (t, 4H, OCH2, J = 4.4 Hz), 7.65–7.78 (m, 3H, ArH), 7.97–8.14 (m, 3H, ArH), 8.35 (s, 1H, ArH), 8.42 (d, 1H, ArH, J = 8.8 Hz), 9.06 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 50.68, 55.98, 115.82, 123.31, 127.10, 127.35, 127.48, 127.54, 127.69, 129.11, 129.17, 130.72, 133.82, 152.00, 165.16; MS: m/z = 427 (M + 1). Anal. calcd. For C22H17F3N4O2; calcd: C, 61.97; H, 4.02; N, 13.14; found: C, 62.00; H, 4.03; N, 13.10%.
N); 1H NMR (400 MHz, DMSO-d6): δ ppm 3.15 (t, 4H, NCH2, J = 4.2 Hz), 4.92 (t, 4H, OCH2, J = 4.4 Hz), 7.65–7.78 (m, 3H, ArH), 7.97–8.14 (m, 3H, ArH), 8.35 (s, 1H, ArH), 8.42 (d, 1H, ArH, J = 8.8 Hz), 9.06 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 50.68, 55.98, 115.82, 123.31, 127.10, 127.35, 127.48, 127.54, 127.69, 129.11, 129.17, 130.72, 133.82, 152.00, 165.16; MS: m/z = 427 (M + 1). Anal. calcd. For C22H17F3N4O2; calcd: C, 61.97; H, 4.02; N, 13.14; found: C, 62.00; H, 4.03; N, 13.10%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6): δ ppm 3.38 (s, 1H, OH), 3.73 (q, 2H, NCH2, J = 5.0 Hz), 3.93 (q, 2H, OCH2, J = 5.0 Hz), 5.08 (s, 1H, NH), 7.65–7.78 (m, 3H, ArH), 7.75 (dd, 1H, ArH, J = 8.9, Hz, J = 1.5 Hz), 8.20–8.22 (m, 2H, ArH), 8.70 (d, 1H, ArH, J = 8.9 Hz), 8.96 (t, 1H, ArH, J = 8.7 Hz), 9.21 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 50.98, 60.32, 118.99, 119.70, 121.44, 123.60, 125.68, 126.99, 127.35, 128.06, 129.07, 130.87, 132.36, 148.92, 150.28, 151.24, 162.92, 163.23. MS: m/z = 401 (M + 1). Anal. calcd. For C20H15F3N4O2; calcd: C, 60.00; H, 3.78; N, 13.99; found: C, 60.03; H, 3.88; N, 13.94%.
N); 1H NMR (400 MHz, DMSO-d6): δ ppm 3.38 (s, 1H, OH), 3.73 (q, 2H, NCH2, J = 5.0 Hz), 3.93 (q, 2H, OCH2, J = 5.0 Hz), 5.08 (s, 1H, NH), 7.65–7.78 (m, 3H, ArH), 7.75 (dd, 1H, ArH, J = 8.9, Hz, J = 1.5 Hz), 8.20–8.22 (m, 2H, ArH), 8.70 (d, 1H, ArH, J = 8.9 Hz), 8.96 (t, 1H, ArH, J = 8.7 Hz), 9.21 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 50.98, 60.32, 118.99, 119.70, 121.44, 123.60, 125.68, 126.99, 127.35, 128.06, 129.07, 130.87, 132.36, 148.92, 150.28, 151.24, 162.92, 163.23. MS: m/z = 401 (M + 1). Anal. calcd. For C20H15F3N4O2; calcd: C, 60.00; H, 3.78; N, 13.99; found: C, 60.03; H, 3.88; N, 13.94%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590 (C
O), 1590 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6): δ ppm 2.45 (s, 3H, CH3), 4.15 (q, 2H, NCH2, J = 5.1 Hz), 4.67 (q, 2H, OCH2, J = 5.0 Hz), 5.21 (s, 1H, NH), 7.64–7.70 (m, 3H, ArH), 8.08–8.13 (m, 3H, ArH), 8.51 (d, 1H, ArH, J = 8.9 Hz), 8.61 (t, 1H, ArH, J = 8.6 Hz), 9.89 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 15.98, 51.00, 60.59, 119.13, 121.45, 123.65, 125.60, 127.00, 127.87, 127.91, 128.10, 130.64, 132.36, 149.08, 162.97, 164.02; MS: m/z = 443 (M + 1). Anal. calcd. For C22H17F3N4O3; calcd: C, 59.73; H, 3.87; N, 12.66; found: C, 59.70; H, 3.81; N, 12.76%.
N); 1H NMR (400 MHz, DMSO-d6): δ ppm 2.45 (s, 3H, CH3), 4.15 (q, 2H, NCH2, J = 5.1 Hz), 4.67 (q, 2H, OCH2, J = 5.0 Hz), 5.21 (s, 1H, NH), 7.64–7.70 (m, 3H, ArH), 8.08–8.13 (m, 3H, ArH), 8.51 (d, 1H, ArH, J = 8.9 Hz), 8.61 (t, 1H, ArH, J = 8.6 Hz), 9.89 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 15.98, 51.00, 60.59, 119.13, 121.45, 123.65, 125.60, 127.00, 127.87, 127.91, 128.10, 130.64, 132.36, 149.08, 162.97, 164.02; MS: m/z = 443 (M + 1). Anal. calcd. For C22H17F3N4O3; calcd: C, 59.73; H, 3.87; N, 12.66; found: C, 59.70; H, 3.81; N, 12.76%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1116 (O–CH3); 1H NMR (400 MHz, DMSO-d6): δ ppm 3.10 (s, 3H, OCH3), 3.55 (s, 6H, OCH3), 6.24 (s, 1H, NH), 7.44–7.52 (m, 4H, ArH), 7.72–7.78 (m, 3H, ArH), 8.13 (s, 1H, ArH), 8.66 (d, 1H, ArH, J = 8.0 Hz), 8.82 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 55.83, 55.89, 119.23, 121.46, 123.32, 124.17, 125.82, 127.33, 127.39, 127.71, 129.11, 129.43, 130.07, 133.81, 152.45, 164.96; MS: m/z = 508 (M + 1). Anal. calcd. For C27H21F3N4O4; calcd: C, 63.90; H, 3.97; N, 8.28; found: C, 63.90; H, 3.97; N, 8.30%.
N), 1116 (O–CH3); 1H NMR (400 MHz, DMSO-d6): δ ppm 3.10 (s, 3H, OCH3), 3.55 (s, 6H, OCH3), 6.24 (s, 1H, NH), 7.44–7.52 (m, 4H, ArH), 7.72–7.78 (m, 3H, ArH), 8.13 (s, 1H, ArH), 8.66 (d, 1H, ArH, J = 8.0 Hz), 8.82 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 55.83, 55.89, 119.23, 121.46, 123.32, 124.17, 125.82, 127.33, 127.39, 127.71, 129.11, 129.43, 130.07, 133.81, 152.45, 164.96; MS: m/z = 508 (M + 1). Anal. calcd. For C27H21F3N4O4; calcd: C, 63.90; H, 3.97; N, 8.28; found: C, 63.90; H, 3.97; N, 8.30%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6): δ ppm 2.86 (s, 3H, CH3), 6.30 (s, 1H, NH), 7.30–7.68 (m, 3H, ArH), 7.78 (t, 2H, ArH, J = 8.2 Hz), 7.96–8.06 (m, 3H, ArH), 8.24 (d, 2H, ArH, J = 8.3 Hz), 8.26 (s,1H, ArH), 8.42 (s, 1H, ArH), 8.61 (d, 1H, ArH, J = 8.0 Hz), 9.35 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 17.06, 118.29, 123.15, 123.92, 125.24, 127.00, 127.23, 127.91, 129.06, 129.37, 130.11, 132.07, 132.10, 132.27, 135.11, 137.46, 151.89, 163.17, 163.82; MS: m/z = 498 (M + 1). Anal. calcd. For C28H18F3N5O; calcd: C, 67.60; H, 3.65; N, 14.08; found: C, 67.65; H, 3.64; N, 14.10%.
N); 1H NMR (400 MHz, DMSO-d6): δ ppm 2.86 (s, 3H, CH3), 6.30 (s, 1H, NH), 7.30–7.68 (m, 3H, ArH), 7.78 (t, 2H, ArH, J = 8.2 Hz), 7.96–8.06 (m, 3H, ArH), 8.24 (d, 2H, ArH, J = 8.3 Hz), 8.26 (s,1H, ArH), 8.42 (s, 1H, ArH), 8.61 (d, 1H, ArH, J = 8.0 Hz), 9.35 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ ppm 17.06, 118.29, 123.15, 123.92, 125.24, 127.00, 127.23, 127.91, 129.06, 129.37, 130.11, 132.07, 132.10, 132.27, 135.11, 137.46, 151.89, 163.17, 163.82; MS: m/z = 498 (M + 1). Anal. calcd. For C28H18F3N5O; calcd: C, 67.60; H, 3.65; N, 14.08; found: C, 67.65; H, 3.64; N, 14.10%.5. X-ray crystallographic study of compound 9a
The X-ray crystallographic analysis of the compound 9a was carried out by fine-focus sealed tube graphite, with approximate dimensions 0.44 mm × 0.20 mm × 0.13 mm, grown from the slow evaporation of a dilute ethanol solution at room temperature. The crystal structure solution was worked out by Bruker SMART APEXII DUO CCD diffractometer. All the atoms were located in different Fourier maps and refined isotropically, using a riding model and all the projections were generated using ORTEP. The details of the crystal data and refinement are shown in Table 5. Also the single crystal image for compound 9a is given in Fig. 2.36| Crystal data | |
|---|---|
| Empirical formula | C23H20F3N5O |
| Formula weight | 439.44 |
| Crystal system | Triclinic |
| Crystal dimension | 0.44 mm × 0.20 mm × 0.13 mm |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a (Å) | 8.5065 (15) |
| b (Å) | 10.2176 (17) |
| c (Å) | 13.709 (3) |
| Volume (Å3) | 1060.0 (4) |
| Angle α, β, γ | 103.840, 98.515, 109.034 |
| Z | 2 |
| Crystal density | 1.377 |
| F000 | 456 |
| μ (mm−1) | 0.11 |
| Temperature (T) | 296 |
| Radiation wavelength (Å) | 0.71073 |
| Radiation type | Mo Kα |
| Radiation source | Fine-focus sealed tube |
| Radiation monochromator | Graphite |
| hmin | −11 |
| hmax | 11 |
| kmin | −13 |
| kmax | 12 |
| lmin | −17 |
| lmax | 17 |
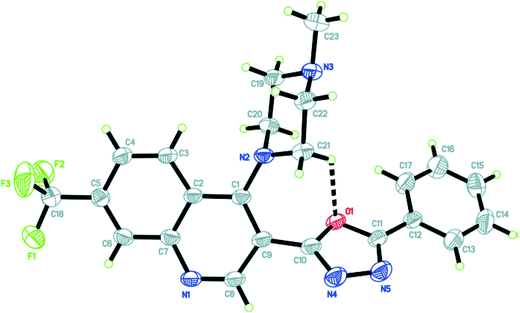 | ||
| Fig. 2 ORTEP diagram showing the X-ray crystal structure of compound 9a.36 | ||
6. Antibacterial studies
The antibacterial activity of newly synthesized compounds (6a–e, 7a–g and 9a–f) were determined by well diffusion method in nutrient agar media.30,31 In vitro antibacterial activity of compounds against 24 h old bacterial culture of a Mycobacterium smegmatis (MTCC 943) and Pseudomonas aeruginosa (MTCC4676) was performed in well diffusion method. Nutrient agar media (about 15–20 millilitres) was poured into each petri plate and allowed to solidify by placing inside the laminar air flow for 15 min. 100 μL of 0.5 McFarland standard of bacterial suspension was inoculated on the agar media and spread on the whole surface with a sterile cotton bud. Using a sterile cork borer, five mm wells were made on the seeded agar plates and 50 μL of test compound at different concentrations (25 and 50 μg mL−1) was transferred in to the wells. The plates were prepared in triplicate for each compound and incubated. Pseudomonas aeruginosa at 30 °C and Mycobacterium smegmatis at 37 °C for 12 h and observed for the zone of inhibition in millimeter. All the compounds are dissolved in DMSO and dilutions of the working solution were made using the same solvent. Ciprofloxacin was used as antibacterial standard.7. Antifungal studies
Antifungal studies of synthesized compounds (6a–e, 7a–g and 9a–f) were carried out against Candida albicans (MTCC 183) and Penicillium chrysogenum (MTCC 6795) using the well diffusion method. Czapek Yeast extract agar media was used for Penicillium chrysogenum and yeast extract peptone dextrose (YEPD) agar media was used for Candida albicans.32,33 Normal saline was used to make a suspension of spore of Penicillium chrysogenum for lawning. A loop full of Penicillium chrysogenum spores was transferred to 3 mL saline to get a spore suspension. Whereas, few colonies of Candida albicans were dispersed in 5 mL of YEPD broth and allowed to grow for few hours before used for spreading the agar plate. Twenty millilitres of agar media was poured into each petri dish and allowed for 15 minutes to solidify and followed the same protocol used for antibacterial activity explained above. Except for the petri plates incubated at 25 °C for 48 hours for Penicillium chrysogenum and 30 °C for Candida albicans for 12–24 hours. Antifungal activity was determined by measuring the diameter of inhibition zone in mm. All the experiments were conducted in triplicates and flucanazole was used as standard antifungal compound.8. MIC against microorganisms
Minimum concentration of compounds required for the inhibition of bacteria M. smegmatis, P. aeruginosa and Staphylococcus aureus and a fungi C. albicans was determined using compound concentration ranging from 1.6–50 μg mL−1. Test compounds are dissolved in DMSO and serially diluted to 50, 25, 12.5, 6.25, 3.125 and 1.6 μg mL−1 of concentration. In a 96 well plate 50 μL of bacterial or fungal suspension was taken and 50 μL of serially diluted above compounds were added to each well and mixed. Incubated the mixture for 12 h and observed each well for the bacterial growth. The lowest concentration at which no microbial growth found was taken as MIC. For the confirmation, 10 μL of the mixture from each well was spread on a nutrient agar plate and incubated to check for any bacterial growth. Each test was repeated in triplicates. Ciprofloxacin and flucanozole were used as standard for bacteria and fungi respectively.Acknowledgements
AMI thanks Department of Atomic Energy, Board for Research in Nuclear Sciences, Government of India for the ‘Young Scientist’ award. Authors thank Department of Information & technology, Government of India, New Delhi for the financial support. Authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through research group project no. RGP-VPP-207.References
- A. M. Isloor, B. Kalluraya and P. Shetty, Eur. J. Med. Chem., 2009, 44, 3784–3787 CrossRef CAS PubMed.
- D. Sunil, A. M. Isloor, P. Shetty, B. Chandrakanth and K. Satyamoorthy, Med. Chem. Res., 2011, 20, 1024–1032 CrossRef CAS.
- A. M. Isloor, D. Sunil, P. Shetty, S. Malladi, K. S. R. Pai and N. Maliyakkl, Med. Chem. Res., 2013, 22, 758–767 Search PubMed.
- U. S. Rai, A. M. Isloor, P. Shetty, A. M. Vijesh, N. Prabhu, S. Isloor, M. Thiageeswaran and H. K. Fun, Eur. J. Med. Chem., 2010, 45, 2695–2699 CrossRef PubMed.
- Y. L. Chen, H. M. Hung, C. M. Lu, K. C. Li and C. C. Tzeng, Bioorg. Med. Chem., 2004, 12, 6539–6546 Search PubMed.
- A. Carta, I. Briguglio, S. Piras, P. Corona, G. Boatto, M. Nieddu, P. Giunchedi, M. E. Marongiu, G. Giliberti, F. Iuliano, S. Blois, C. Ibba, B. Busonera and P. O. Colla, Bioorg. Med. Chem., 2011, 19, 7070–7084 Search PubMed.
- A. A. Bekhit, O. A. El-Sayed, E. Aboulmagd and J. Y. Park, Eur. J. Med. Chem., 2004, 39, 249–255 CrossRef CAS PubMed.
- N. B. Patel and S. D. Patel, Med. Chem. Res., 2010, 19, 757–770 Search PubMed.
- S. Eswaran, A. V. Adhikari and N. S. Shetty, Eur. J. Med. Chem., 2009, 44, 4637–4647 CrossRef CAS PubMed.
- B. S. Jayashree, S. Thomas and Y. Nayak, Med. Chem. Res., 2010, 19, 193–209 Search PubMed.
- S. Vandekerckhove, S. De Moor, D. Segers, C. de Kock, P. J. Smith, K. Chibale, N. De Kimpe and M. D'hooghe, MedChemComm, 2013, 4, 724–730 RSC.
- K. D. Thomas, A. V. Adhikari, S. Telkar, I. H. Chowdhury, R. Mahmood, N. K. Pal, G. Row and E. Sumesh, Eur. J. Med. Chem., 2011, 46, 5283–5292 Search PubMed.
- S. R. Hawley, P. G. Bray, P. M. O'Neill, B. K. Park and S. A. Ward, Biochem. Pharmacol., 1996, 52, 723–733 Search PubMed.
- B. S. Holla, M. Mahalinga, M. S. Karthikeyan, P. M. Akbarali and N. S. Shetty, Bioorg. Med. Chem., 2006, 14, 2040–2047 CrossRef CAS PubMed.
- J. He, L. Yan, R. Yang, Z. Xiao, J. Cheng, W. Zhou and Y. Zhang, Bioorg. Med. Chem. Lett., 2005, 15, 2980–2985 CrossRef CAS PubMed.
- Y. Bi, P. Stoy, L. Adam, B. He, J. Krupinski, D. Normandin, R. Pongrac, L. Seliger, A. Watson and J. E. Macora, Bioorg. Med. Chem. Lett., 2004, 14, 1577–1580 CrossRef CAS PubMed.
- K. A. Metwally, L. M. Abdel-Aziz, E. M. Lashine, M. I. Husseiny and R. H. Badawy, Bioorg. Med. Chem., 2006, 14, 8675–8682 CrossRef CAS PubMed.
- C. Fattorusso, G. Campiani, G. Kukreja, M. Persico, S. Butini, M. P. Romano, M. Altarelli, S. Ros, M. Brindisi, L. Savini, E. Novellino, V. Nacci, E. Fattorusso, S. Parapini, N. Basilico, D. Taramelli, V. Yardley, S. Croft, M. Borriello and S. Gemma, J. Med. Chem., 2008, 51, 1333–1343 CrossRef CAS PubMed.
- E. Vavrikova, S. Polanc, M. Kocevar, K. Horvati, S. Bosze, J. Stolarikova, K. Vavrova and J. Vinsova, Eur. J. Med. Chem., 2011, 46, 4937–4945 CrossRef CAS PubMed.
- B. Chandrakantha, P. Shetty, V. Nambiyar, N. Isloor and A. M. Isloor, Eur. J. Med. Chem., 2010, 45, 1206–1210 Search PubMed.
- C. Chen, B. Song, S. Yang, G. Xu, P. S. Bhadury, L. Jin, D. Hu, Q. Li, F. Liu, W. Xue, P. Lu and Z. Chen, Bioorg. Med. Chem., 2007, 15, 3981–3989 CrossRef CAS PubMed.
- G. C. Ramaprasad, B. Kalluraya, B. S. Kumar and R. K. Hunnur, Eur. J. Med. Chem., 2010, 45, 4587–4593 CrossRef CAS PubMed.
- Y. Cheng, B. K. Albrecht, J. Brown, J. L. Buchanan, W. H. Buckner, E. F. DiMauro, R. Emkey, R. T. Fremeau, J. Harmange, B. J. Hoffman, L. Huang, M. Huang, J. H. Lee, F. Lin, M. W. Martin, H. Q. Nguyen, V. F. Patel, S. A. Tomlinson, R. D. White, X. Xia and S. A. Hitchcock, J. Med. Chem., 2008, 51, 5019–5034 CrossRef CAS PubMed.
- L. Eugene, P. Chekler, A. S. Kiselyov, X. Ouyang, X. Chen, V. Pattaropong, Y. Wang, M. C. Tuma and J. F. Doody, ACS Med. Chem. Lett., 2010, 1, 488–492 CrossRef PubMed.
- B. Garudachari, M. N. Satyanarayana, B. Thippeswamy, C. K. Shivakumar, K. N. Shivananda and A. M. Isloor, Eur. J. Med. Chem., 2012, 54, 900–906 CrossRef CAS PubMed.
- B. Garudachari, A. M. Isloor, M. N. Satyanarayana, H.-K. Fun, N. Pavithra and A. Kulal, J. Med. Chem., 2013, 68, 422–432 CrossRef CAS PubMed.
- H. R. Snyder, H. E. Freier, P. Kovacic and E. M. V. Heyningen, J. Am. Chem. Soc., 1947, 69, 371–374 CrossRef CAS.
- A. Kumar, B. P. Jaju, S. Gurutu, J. N. Sinha and K. Shanker, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1988, 27, 93–95 Search PubMed.
- M. A. Kira, M. O. Abdel-Rahman and K. Z. Gadalla, Tetrahedron Lett., 1969, 10, 109–110 CrossRef.
- L. Rocha, A. Marston, O. Potterat, M. A. C. Kaplan, H. Stoeckli-Evans and K. Hostettmann, Phytochemistry, 1995, 40, 1447–1452 CrossRef CAS.
- B. A. Arthington-Skaggs, M. Motley, D. W. Warnock and C. J. Morrison, J. Clin. Microbiol., 2000, 38, 2254–2260 CAS.
- D. J. Mac-Lowry, M. J. Jaqua and S. T. Selepak, Appl. Microbiol., 1970, 20, 46–53 Search PubMed.
- A. Portillo, R. Vila, B. Freixa, T. Adzet and S. Can-igueral, J. Ethnopharmacol., 2001, 76, 93–98 CrossRef CAS.
- J. T. Jaouhari, H. B. Lazrek, A. Seddik and M. Jana, J. Ethnopharmacol., 1999, 64, 211–217 Search PubMed.
- M. N. Ghosh, Scientific Book Agency, Calcutta, India, 2nd edn, 1984 Search PubMed.
- H. K. Fun, S. Arshad, B. Garudachari, A. M. Isloor and M. N. Satyanarayan, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2011, 67, 3117–3118 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra04456h |
| This journal is © The Royal Society of Chemistry 2014 |