Novel bio-based composites of polyhydroxyalkanoate (PHA)/distillers dried grains with solubles (DDGS)
Abstract
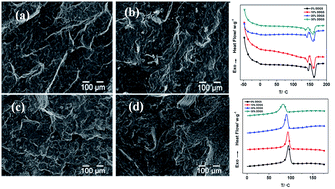
The PHA/DDGS composite is a promising low-cost, bio-based material for use in crop containers for the horticulture industry. This research effort has quantified the effects on mechanical and thermal properties of adding different amounts of DDGS to a PHA matrix. PHA and DDGS were mixed using a twin-screw microcompounder. Fracture surface morphology and thermal and rheological properties were evaluated using scanning electron microscopy (SEM), thermogravimetric analysis (TGA), dynamic mechanical analysis (DMA), differential scanning calorimetry (DSC), and rheometer measurements. The adhesion between PHA and DDGS decreased with an increase in DDGS content from 10% to 30%. Melting temperature and crystalline temperature decreased with the increasing content of DDGS filler, indicating that PHA and DDGS interacted favorably. The complex viscosity and elastic shear modulus of the blends were increased by the increasing DDGS content. The storage modulus and glass transition temperature showed little change across the different ratios of DDGS, indicating that DDGS should be a useful filler that can decrease the cost of PHA-based materials significantly while preserving the dynamic mechanical properties and glass transition temperature.

 Please wait while we load your content...
Please wait while we load your content...