Carbon-protected Au nanoparticles supported on mesoporous TiO2 for catalytic reduction of p-nitrophenol†
Abstract
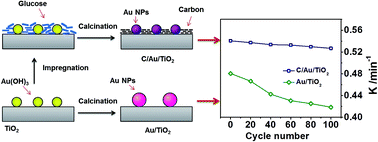
With the introduction of carbon on Au/TiO2, the reaction rate of C/Au/TiO2 increased by 29% and the stability was enhanced by about 3 times more than Au/TiO2 in the p-nitrophenol reduction reaction. Carbon species enhanced the stability of Au nanoparticles and also increase the organic reactants adsorptive ability.

 Please wait while we load your content...
Please wait while we load your content...