Aqueous extract of Balanites roxburghii fruit: a green dispersant for C–C bond formation
Madhuri Barge and
Rajashri Salunkhe*
Department of Chemistry, Shivaji University, Kolhapur, 416004, Maharashtra, India. E-mail: rsschem1@gmail.com; Fax: +91 231 2692333; Tel: +91 231 2609240
First published on 3rd July 2014
Abstract
An aqueous biosurfactant solution, a biobased green acidic catalyst for Knoevenagel condensation of 1,3-indanedione with aryl aldehydes and tandem Knoevenagel–Michael reaction of 3-methyl-1-phenylpyrazole with aryl aldehydes, has been reported for the first time. The synthetic pathway complies with several key requirements of green chemistry principles such as the use of renewable feedstock, aqueous conditions, waste prevention, atom economy along with the biodegradable and recyclable catalyst. Thus the presented protocol offers an attractive option because of its versatility, ecological safety, environmental acceptance and sustainability.
Introduction
Recently energy efficient technologies eliminating waste production, toxic and hazardous solvents and reagents as well as employing renewable raw materials are experiencing a profound challenge to meet sustainability criteria.1 In addition the environmental risks posed by the volatile organic solvents have become a major concern. The reason is that the organic transformations employ higher consumption of solvents than the reagents and the employed solvents are difficult to recycle.2 For the process to be in line with the green chemistry principles, the first task is to replace the volatile organic solvents with green ones.An ideal solvent that meets all the goals of function, performance, economy and greenness is water, which has witnessed increasing popularity due to being inexpensive, readily available, non-inflammable, non-toxic, safe and environmentally benign.3 However, organic reactions in water are often limited in scope due to the scarce solubility of many of the organic compounds. A possible new way to improve the solubility of organic substrates is the use of amphiphiles such as surfactants,4 hydrotropes5 and phase transfer catalysts.6
Nowadays biosynthetic processes involving biobased solvents or catalysts have received much attention as a viable alternative for the development of green methodologies for organic synthesis.7 In this regard, biosurfactants or natural dispersants as a part of the chemical process offer an excellent alternative to volatile organic solvents in being more environmental friendly technologies due to their low toxicity, ease of biodegradability, ability to act as catalyst, non-flammability and non-corrosive properties as compared to petroleum-based surfactants.8 Also, due to the high natural abundance their production is potentially less expensive.
Biosurfactants (surface active agents) are microbial amphiphilic polymers obtained from plants, animals or microorganisms. They form part of an emergent tool with a great potential for industrial applications including the use in enhanced oil recovery, crude oil drilling, lubricants, health care and food processing industry.9 Also full evaluations of the potential of these natural dispersants in cosmetic and soap formulations, foods and dermal or transdermal drug delivery systems are developing at an incredible rate.10 Notably, despite their diverse applications in industry and environmental biotechnology, their potential in accelerating the organic transformations has not been evolved till date. Therefore the aim of present work is to explore the synthetic utility of natural sugar-based surfactant in organic transformations. The catalytic medium is sourced from the aqueous extract of Balanites roxburghii fruit. From literature records it is well known that the Balanites roxburghii fruit locally known as Hingota in India is a wild plant species traditionally used as emetic, anthelmintic, anti-fungal, and purgative, in whooping cough, skin diseases and snake bite. Phytochemical study showed the presence of alkaloids, flavonoids, tannins, phenolic compounds and saponins.11 In addition to this, aqueous fruit extract exhibits acidic pH (ca. 4.86). In view of this data and in continuation of our ongoing research in the development of green synthetic routes,13 we thought this amazing medium may serve as a better alternative to chemical surfactants and also to harmful corrosive acids.
Results and discussion
Initially, we focused our attention to prepare dispersant solution from Balanites roxburghii fruit. For this purpose mesocarp of a dried fruit was crushed to get powder which weighted about 3–4 g. The powder (1 g) was soaked in distilled water (100 mL) for 12 hours. The resultant material was then macerated and filtered to give wine red coloured solution.The next task was to optimise different reaction parameters for Knoevenagel reaction. Knoevenagel products i.e. 2-arylideneindane-1,3-dione scaffolds obtained from 1,3-indanedione and aryl aldehydes are industrially important precursors because they are used as intermediates for the synthesis of different bio-active molecules.14 As well as they own propitious pharmacological activities such as anticoagulants15 and cytotoxics.16 Only sporadic reports are available for the synthesis of these derivatives. A detailed literature survey about the synthesis of these derivatives revealed that most of the methods employed operated under reflux conditions using various catalysts including acids or bases such as acetic acid, concentrated H2SO4,15 gaseous HCl and p-TSA,17 piperidine.18 Ren et al. reported solvent-free route by grinding method in presence of silica gel and MgO as catalysts at ambient temperature.19 Recently, Yang et al. reported catalyst-free synthesis of 2-arylideneindane-1,3-diones in refluxing water but the process is very tedious and lengthy.20 Thus, the generality of the existing reports is somewhat visited by the severe reaction conditions and the catalysts and solvents used are not acceptable in the context of green synthesis. To defeat these circumstances we employed a simple and efficient micellar approach which led to the development of new, rapid and robust route towards focused libraries.
To optimize the reaction conditions, a round bottom flask was charged with 1,3-indanedione 1 (1 mmol) and 2-nitrobenzaldehyde 2h (1 mmol). To this, aqueous fruit extract (5 mL) was added and stirred the mixture at room temperature. On the completion of reaction as monitored by TLC, the product separated out by simple filtration which on sufficient washing with water and ethanol afforded the corresponding product of high purity.
To check the effect of dilution on the yield of product, the model reaction was carried out with various concentrations of dispersant solution. Initially prepared wine coloured solution was diluted with distilled water till to get a clear solution. It was surprising to note that there was no effect on the yield of product even with the much diluted clear solution (Table 1, entry 1 and 2).
| Entry | Catalyst | Time (min) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1,3-indanedione (1 mmol), 2-nitrobenzaldehyde (1 mmol), rt.b Isolated yields. | |||
| 1 | Wine red coloured surfactant solution (5 mL) | 5 | 94 |
| 2 | Diluted colourless surfactant solution (5 mL) | 5 | 94 |
| 3 | SDS (10 mol%) | 18 | 86 |
| 4 | Sodium stearate (10 mol%) | 25 | 79 |
| 5 | Diluted colourless surfactant solution (fifth run) | 5 | 94 |
| 6 | Distilled water (5 mL) | 6 h | — |
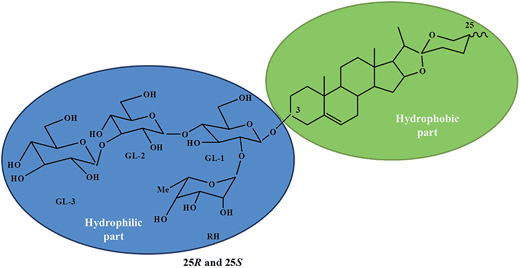
To understand this effect, pH of each solution was measured and amazingly it was observed that pH remained constant for each diluted solution which indicated that the aqueous extract of Balanites roxburghii fruit worked like buffer. Furthermore, addition of acid or base could not change the pH of catalytic medium confirming the buffering property of medium. Buffering action of the catalytic solution is accounted on the basis of structure of one of the saponins12 (Fig. 1) present in fruit extract. Saponin is an amphiphilic molecule in which sugar is linked to sterol non-polar group. As we know sugar part acts as a buffer and thus change in concentration by dilution with water or by addition of acid or base didn't affect the catalytic property of biosurfactant solution.
Thus acidic nature of aqueous extract as well as surface activity due to saponins offered synergistic effect and reaction proceeded rapidly within short time. To compare the catalytic activity of natural surfactant with chemical surfactant, we also carried out the model reaction using synthetic surfactants (Table 1, entry 3 and 4). Natural surfactant was found to be excellent alternative to the chemical surfactants with respect to time as well as yield of the product. In addition biosurfactant is a recyclable buffer system which furnished the constant yield of product for several runs (Table 1, entry 5) indicating its versatile reusability which is seldom possible for chemical surfactants. A controlled reaction conducted under identical conditions and devoid of biosurfactant only in water could not furnish the corresponding product, despite the prolonged reaction times indicates role of biosurfactant is decisive (Table 1, entry 6).
After the optimization of concentration, a series of structurally diverse aryl aldehydes were treated with 1,3-indanedione in diluted dispersant solution at ambient temperature (Table 2). The reactions proceeded at room temperature within 5 to 20 minutes affording the desired products in excellent yields. The aryl aldehydes bearing electron-donating as well as electron-withdrawing groups underwent reactions successfully. The method is also suitable for the sterically hindered 1-naphthaldehyde (Table 2, entries 10). In addition, heteroaromatic aldehydes such as furfuraldehyde and thiophene-2-aldehyde (Table 2, entries 14 and 15) reacted efficiently furnishing anticipated products in good yields. In all the cases 2-arylideneindane-1,3-diones were the sole products and no anomalies were noted. Pure products obtained by washing with water and hot ethanol were characterised by their physical constants and spectral techniques.
| Sr. no. | Aldehyde 2(a–r) | Producta 3(a–r) | Time (min) | Yieldb (%) | mp [Lit.]c (°C) |
|---|---|---|---|---|---|
| a Representative products were characterized by IR, 1H NMR, 13C NMR and mass spectroscopy as well as elemental analysis technique.b Isolated yields.c Literature values in parenthesis. | |||||
| 1 | Ph | 3a | 5 | 93 | 150 [152–153]15 |
| 2 | 4-Me-C6H4 | 3b | 15 | 92 | 150 [150–151]19 |
| 3 | 4-OMe-C6H4 | 3c | 20 | 89 | 155 [156–157]19 |
| 4 | 4-Cl-C6H4 | 3d | 5 | 92 | 180 [180–182]20 |
| 5 | 4-F-C6H4 | 3e | 5 | 90 | 170 [170]18 |
| 6 | 2-OH-C6H4 | 3f | 12 | 88 | 194 [193–195]20 |
| 7 | 4-OH-C6H4 | 3g | 10 | 90 | 241 [241–243]20 |
| 8 | 2-NO2-C6H4 | 3h | 5 | 94 | 190 [192–194]20 |
| 9 | 4-NO2-C6H4 | 3i | 17 | 94 | 232 [234–236]20 |
| 10 | 1-Naphthyl | 3j | 10 | 83 | 172 [174–176]15 |
| 11 | 4-N(Me)2-C6H4 | 3k | 20 | 88 | 178 [180]18 |
| 12 | 4-OH, 3-OMe-C6H3 | 3l | 15 | 93 | 218–220 |
| 13 | 3,4,5-(OMe)3-C6H2 | 3m | 18 | 92 | 185 [185]18 |
| 14 | Furyl-2-yl | 3n | 5 | 90 | 210 [209–211]20 |
| 15 | Thiophene-2-yl | 3o | 5 | 94 | 178–180 |
| 16 | 2-CHO-C6H4 | 3p | 5 | 92 | 218–220 |
| 17 | 4-Br-C6H4 | 3q | 5 | 90 | 173–175 |
| 18 | 4-CN-C6H4 | 3r | 5 | 93 | 238–240 |
Inspired by these tempting results obtained for Knoevenagel condensation at ambient temperature, we extended same protocol for Knoevenagel–Michael reaction of aryl aldehydes and 3-methyl-1-phenyl-5-pyrazolone to yield 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols). This is because pyrazolone nucleus is one of the most omnipresent heterocyclic structures found in numerous bioactive molecules with predominant properties such as antitumor,21 selective COX-2 inhibitory22 and cytokine inhibitors.23 They found many applications as extractant for metal ions and ligands.24 2,4-Dihydro-3H-pyrazol-3-one derivatives including 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) also display a wide range of pharmacological activities such as gastric secretion stimulatory,25 antidepressant,26 antibacterial27 and antifilarial agents.28 Also, they are extensively applied as fungicides, pesticides, insecticides and dyestuffs.29
Synthetic route to prepare 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) generally involves the tandem Knoevenagel–Michael reaction of aryl aldehydes with two equivalents of 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one. Several methods and catalysts have been reported in literature to synthesize these derivatives including microwave irradiation,30 triethylbenzylammonium chloride (TEBA),31 sodium dodecyl sulphate,32 electrolysis,33 cerium(IV) ammonium nitrate,34 silica bonded S-sulfonic acid,35 cellulose sulphuric acid,36 silica sulfuric acid,37 silica bonded ionic liquid38 and 1,3-disulfonic acid imidazolium tetrachloroaluminate {[Dsim]AlCl4}.39 Although diverse approaches towards the synthesis of these derivatives have been developed, use of biosurfactant is the most elegant strategy.
The condensation reactions were carried out in 5 mL aqueous extract of fruit at 60 °C in a preheated oil bath using a series of structurally diverse aryl aldehydes with 3-methyl-1-phenyl-5-pyrazolone (Table 3). Initially Knoevenagel condensation proceeded rapidly within 2 minutes to furnish 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 products i.e. orange coloured arylidenepyrazolones which were converted to final white products through Michael step within 15–60 minutes. On the completion of reactions as monitored by TLC, the reaction mixtures were filtered to isolate products and purified by washing with water and hot ethanol. In IR spectrum of all the compounds characteristic broad absorption in the region 2500–2600 cm−1 was noticed for the H-bonded enolic –OH and carbonyl absorption was absent indicating that the pyrazolonyl group in all compounds exists in enol form.40
1 products i.e. orange coloured arylidenepyrazolones which were converted to final white products through Michael step within 15–60 minutes. On the completion of reactions as monitored by TLC, the reaction mixtures were filtered to isolate products and purified by washing with water and hot ethanol. In IR spectrum of all the compounds characteristic broad absorption in the region 2500–2600 cm−1 was noticed for the H-bonded enolic –OH and carbonyl absorption was absent indicating that the pyrazolonyl group in all compounds exists in enol form.40
| Sr. no. | Aldehyde 5(a–m) | Producta 6(a–m) | Time (min) | Yieldb (%) | mp [Lit.]c (°C) |
|---|---|---|---|---|---|
| a Representative products were characterized by IR, 1H NMR, 13C NMR spectroscopy and elemental analysis technique.b Isolated yields.c Literature values in parenthesis. | |||||
| 1 | Ph | 6a | 15 | 90 | 170–171 [169–171]32 |
| 2 | 4-Me-C6H4 | 6b | 20 | 93 | 200–202 [203]32 |
| 3 | 4-OMe-C6H4 | 6c | 30 | 90 | 148–150 [148]40b |
| 4 | 2-Cl-C6H4 | 6d | 20 | 92 | 235–236 [235–237]32 |
| 5 | 4-Cl-C6H4 | 6e | 20 | 90 | 215–217 [214–217]39 |
| 6 | 4-Br-C6H4 | 6f | 25 | 88 | 215–217 [215]40b |
| 7 | 4-F-C6H4 | 6g | 25 | 85 | 180–182 [182]40b |
| 8 | 4-NO2-C6H4 | 6h | 25 | 83 | 218–220 [218–220]32 |
| 9 | 2-NO2-C6H4 | 6i | 30 | 89 | 220–222 [221–222]32 |
| 10 | 3-NO2-C6H4 | 6j | 50 | 86 | 151–153 [151–153]32 |
| 11 | Thiophene-2-yl | 6k | 25 | 89 | 187–189 [189–190]39 |
| 12 | 2-CHO-C6H4 | 6l | 35 | 90 | 247–250 |
| 13 | 1-Naphthyl | 6m | 60 | 87 | 208–210 |
Since the blank reaction only in water could not give the corresponding products even after the long reaction time, the exceptionally higher catalytic activity of biosurfactant can be related to its ability to form micelles in water. The role of micelle to catalyse the reaction can be explained as shown in Fig. 2(a). As the impact of micellar solution, hydrophobic reactants i.e. pyrazolone 4 and aldehyde 5 get pushed away from water molecules towards the hydrophobic core of micelle leading to the effective collisions and water formed by condensations is repelled out to give corresponding Knoevenagel product 7 which was further reacted with another molecule of pyrazolone with shifting of equilibrium towards formation of desired product 6 with excellent yield. As the impact of micellar solution reaction mixture turned turbid due to the formation of colloidal aggregates which was confirmed on the basis of optical microscopy (Fig. 2(b)).
 | ||
| Fig. 2 (a) Mechanistic picture of bispyrazolone formation. (b) Optical micrograph of reaction mixture. | ||
Conclusion
In summary, we report a straightforward bioorganic approach for C–C bond construction via Knoevenagel condensation and tandem Knoevenagel–Michael reaction which represents a highly efficient and environmental benign system. Apart from this simplicity of product separation and effortless reusability of catalyst are the significant advantages of this protocol. This new acidic dispersant should thereby provide attractive alternative to the harmful corrosive acids. However in true sense, we think that biosurfactant doesn't offer only a synergistic effect of acidic nature and surface activity but might be combination of many other factors. Hence to have a good contribution in innovation of green chemistry, we will lengthen our research to have more exposure to natural dispersant mediated organic transformations.Experimental
Solvents and reagents were commercially sourced from Sigma Aldrich and used without further purification. Melting points were determined in an open capillary and are uncorrected. Infrared spectra were measured with a Perkin Elmer FT-IR spectrophotometer. The samples were examined as KBr discs ∼5% w/w. 1H NMR and 13C NMR spectra were recorded on a Bruker AC (300 MHz for 1H NMR and 75 MHz for 13C NMR) spectrometer using CDCl3 and DMSO-d6 as solvents. Chemical shifts are expressed in δ parts per million (ppm) values with tetramethylsilane (TMS) as the internal reference and coupling constants are expressed in Hertz (Hz). Mass spectra were recorded on Shimadzu QP2010 GCMS. Elemental analysis was carried out using Euro EA 3000 Vectro model. Optical micrograph was taken using ordinary light microscope (Leica DM 2000) under 100× magnifications.Preparation of aqueous extract from plant material
Dried fruits of Balanites roxburghii plant were purchased from local market and authenticated by the Department of Botany, Shivaji University, Kolhapur, India. The mesocarp of a fruit was crushed to get powder weighted about 3–4 g. 1 g powder was soaked in 100 mL of distilled water for 12 hours and filtered to get wine red coloured solution. This concentrated solution was diluted by distilled water till to get colourless solution. Dispersant solution was stored below 5 °C and found to be stable for several months.General procedure for synthesis of 2-arylideneindane-1,3-diones
A mixture of 1,3-indanedione (1 mmol) and aldehyde (1 mmol) in biosurfactant (5 mL) was stirred at ambient temperature till the completion of reaction as indicated by TLC (pet. ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate, 7
ethyl acetate, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The solid products were separated by adding 50 mL water followed by simple filtration. The pure products were obtained by washing with water and hot ethanol. The identity of representative compounds was ascertained on the basis of IR, 1H NMR, 13C NMR spectroscopy and mass spectrometry as well as by elemental analysis. The physical and spectroscopic data are in consistent with the proposed structures and are in harmony with the literature values.
3). The solid products were separated by adding 50 mL water followed by simple filtration. The pure products were obtained by washing with water and hot ethanol. The identity of representative compounds was ascertained on the basis of IR, 1H NMR, 13C NMR spectroscopy and mass spectrometry as well as by elemental analysis. The physical and spectroscopic data are in consistent with the proposed structures and are in harmony with the literature values.
General procedure for synthesis of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s
In a 100 mL round bottom flask 3-methyl-l-phenyl-5-pyrazolone (2 mmol) and aldehyde (1 mmol) were placed in biosurfactant solution (5 mL) and stirred at 60 °C temperature in preheated oil bath till the completion of reaction as indicated by TLC (pet. ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate, 8
ethyl acetate, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The solid products were separated by simple filtration and purified by washing with water and hot ethanol. Representative compounds were confirmed by physical constants and characterized by spectral analysis.
2). The solid products were separated by simple filtration and purified by washing with water and hot ethanol. Representative compounds were confirmed by physical constants and characterized by spectral analysis.
Spectral data of representative compounds
2-(4-Chlorobenzylidene)-2H-indene-1,3-dione (Table 2, entry 3d): white solid; mp 180 °C; IR (KBr): νmax/cm−1 = 1726 and 1690 (CO), 1580, 831, 736; 1H NMR (300 MHz, CDCl3): δ = 7.49–7.52 (d, 2H), 7.84–7.88 (m, 3H), 8.02–8.06 (m, 2H), 8.44–8.46 (d, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ = 123.4, 123.4, 129.1, 129.5, 131.7, 135.3, 135.5, 139.5, 140.5, 143.0, 145.1, 189.1 (CO), 190.3 (CO) ppm; elemental analysis: requires: C, 71.52; H, 3.38; O, 11.91% (C16H9O2Cl) found: C, 71.49; H, 3.40; O, 11.97%.2-(4-Hydroxy, 3-methoxybenzylidene)-2H-indene-1,3-dione (Table 2, entry 3l): yellow solid; mp 218–220 °C; IR (KBr): νmax/cm−1 = 3453 (OH), 1715 and 1672 (CO), 998, 740 cm−1; 1H NMR (300 MHz, CDCl3): δ = 4.15 (s, 3H), 6.27 (s, 1H), 7.00–7.03 (d, 1H), 7.60–7.64 (dd, 1H), 7.78–7.83 (m, 3H), 7.97–8.01 (m, 2H), 9.04 (d, 1H) ppm; 13C NMR (75 MHz, CDCl3): δ = 56.1, 114.5, 115.1, 122.9, 123.1, 126.1, 126.6, 132.2, 134.6, 134.8, 140.0, 142.4, 146.3, 147.7, 151.2, 189.6 (CO), 190.3 (CO) ppm; elemental analysis: requires: C, 72.85; H, 4.32; O, 22.83% (C16H10O3) found: C, 72.80; H, 4.38; O, 22.84%.
2-((Thiophen-2-yl)methylene)-2H-indane-1,3-dione (Table 2, entry 3o): yellow solid; mp 178–180 °C; IR (KBr): νmax/cm−1 = 1724 and 1684 (CO), 1585, 811, 726 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.25–7.27 (m, 1H), 7.79–7.83 (m, 1H), 7.87–7.88 (d, 1H), 7.98–8.06 (m, 3H), 8.10–8.11 (d, 1H) ppm; 13C NMR (75 MHz, CDCl3): δ = 123.0, 123.1, 124.9, 128.5, 134.7, 134.9, 136.0, 137.5, 137.9, 140.4, 141.3, 142.1, 188.9 (CO), 189.7 (CO) ppm; elemental analysis: requires: C, 69.98; H, 3.36; O, 13.32% (C14H8O2S) found: C, 69.96; H, 3.40; O, 13.35%.
2-((1,3-Dioxo-1H-inden-2(3H)-ylidene)methyl)benzaldehyde (Table 2, entry 3p): white solid; mp 218–220 °C; IR (KBr): νmax/cm−1 = 1723, 1710 and 1691 (CO), 1589, 805, 733 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.69–7.74 (t, 1H), 7.85–7.90 (m, 2H), 7.94–7.98 (m, 1H), 8.03–8.11 (m, 3H), 8.67–8.69 (d, 1H), 9.03 (s, 1H), 10.16 (s, 1H) ppm; 13C NMR (75 MHz, CDCl3): δ = 123.5, 123.6, 129.4, 130.6, 132.2, 133.8, 135.3, 135.4, 135.8, 136.9, 139.0, 140.2, 142.6, 144.5, 188.4 (CO), 189.1 (CO), 190.8 (CO) ppm; mass (EI): m/z = 262; elemental analysis: requires: C, 77.85; H, 3.84; O, 18.30% (C17H10O3) found: C, 77.88; H, 3.80; O, 18.31%.
2-(4-Bromobenzylidene)-2H-indene-1, 3-dione (Table 2, entry 3q): yellow solid; mp 173–175 °C; IR (KBr): νmax/cm−1 = 1725 and 1689 (CO), 1578, 991, 736 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.66–7.88 (d, 2H), 7.82–7.88 (m, 3H), 8.02–8.05 (m, 2H), 8.35–8.38 (d, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ = 123.4, 123.5, 128.3, 129.6, 131.9, 132.1, 135.2, 135.3, 135.4, 140.1, 142.6, 145.1, 188.7 (CO), 189.6 (CO) ppm; elemental analysis: require: C, 61.37; H, 2.90; O, 10.22% (C16H9O2Br) found: C, 61.41; H, 2.91; O, 10.19%.
2-(4-Cyanobenzylidene)-2H-indene-1, 3-dione (Table 2, entry 3r): yellow solid; mp 238–240 °C; IR (KBr): νmax/cm−1 = 2227 (CN), 1727 and 1689 (CO), 1589, 989, 733 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.80–7.92 (m, 5H), 8.05–8.08 (q, 2H), 8.51–8.54 (d, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ = 115.5, 117.9, 123.6, 123.7, 131.8, 132.2, 133.7, 135.6, 135.7, 136.6, 140.2, 142.6, 143.2, 188.2 (CO), 188.9 (CO) ppm; mass (EI): m/z = 282; elemental analysis: requires: C, 78.76; H, 3.50; O, 12.34% (C17H9NO2) found: C, 78.80; H, 3.52; O, 12.32%.
4,4′-(Phenylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) (Table 3, entry 6a): pale yellow solid; mp 170–172 °C; IR (KBr): νmax/cm−1 = 2620 (OH), 1615, 1489, 780 cm−1; 1H NMR (300 MHz, DMSO-d6): δ = 2.30 (s, 6H, CH3), 4.98 (s, 1H, CH), 7.12–7.16 (m, 1H, ArH), 7.21–7.25 (m, 6H, ArH), 7.38–7.42 (t, 4H, ArH), 7.58–7.62 (d, 4H, ArH) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 12.0, 33.5, 121.3, 126.4, 126.5, 127.7, 128.7, 129.5, 137.4, 142.5, 146.8 ppm; elemental analysis requires: C, 74.29; H, 5.54; O, 7.33% (C27H24 N4O2) found: C, 74.31; H, 5.50; O, 7.38%.
4,4′-[(4-Chlorophenyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) (Table 3, entry 6e): white solid; mp 215–217 °C; IR (KBr): νmax/cm−1 = 2553 (OH), 1598 cm−1; 1H NMR (300 MHz, DMSO-d6): δ = 2.31 (s, 6H, CH3), 4.86 (s, 1H, CH), 7.18–7.21 (d, 6H, ArH), 7.34–7.39 (t, 4H, ArH), 7.69–7.02 (d, 4H, ArH), 13.69 (brs, 1H, OH) ppm; 13C NMR (75 MHz, CDCl3): δ = 11.8, 3 3.1, 120.9, 125.6, 128.2, 128.8, 129.1, 131.5, 137.6, 140.7, 145.9 ppm; elemental analysis: requires: C, 68.86; H, 4.92; O, 6.79% (C27H23N4O2Cl) found: C, 68.80; H, 4.94; O, 6.75%.
4,4′-[(2-Thienyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) (Table 3, entry 6k): off white solid; mp 190–193 °C; IR (KBr): νmax/cm−1 = 2600, 1525 cm−1; 1H NMR (300 MHz, DMSO-d6): δ = 2.32 (s, 6H, CH3), 5.04 (s, 1H, CH), 6.74–6.75 (d, 4H, ArH), 6.84 (s, 1H, ArH), 7.17–7.22 (t, 3H, ArH), 7.37–7.41 (t, 3H, ArH), 13.88 (brs, 1H, OH) ppm; 13C NMR (75 MHz, CDCl3): δ = 11.8, 30.7, 120.8, 124.0, 124.9, 125.7, 126.9, 128.9, 145.7, 147.6 ppm; elemental analysis: requires: C, 67.85; H, 5.01; O, 7.23% (C25H22N4O2S) found: C, 67.80; H, 5.05; O, 7.27%.
4,4′-[(2-Formyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) (Table 3, entry 6l): white solid; mp 247–250 °C; IR (KBr): νmax/cm−1= 2552 (OH), 1688 (CO), 1598, cm−1; 1H NMR (300 MHz, DMSO-d6): δ = 2.34 (s, 6H, CH3), 4.96 (s, 1H, CH), 7.15–7.20 (m, 2H, ArH), 7.34–7.47 (m, 4H, ArH), 7.57–7.59 (d, 2H, ArH), 7.70–7.73 (m, 6H), 9.92 (s, 1H), 13.80 (brs, 1H, OH) ppm; 13C NMR (75 MHz, CDCl3): δ = 11.9, 33.5, 104.7, 120.9, 125.7, 127.8, 128.5, 128.9, 129.1, 133.8, 136.5, 137.6, 143.4, 146.1, 157.6, 192.6 ppm; elemental analysis: requires: C, 72.40; H, 5.21; O, 10.33% (C28H24N4O3) found: C, 72.44; H, 5.18; O, 10.37%.
4,4′-[(1-Naphthyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) (Table 3, entry 6m): white solid; mp 208–210 °C; IR (KBr): νmax/cm−1 = 2585 (OH), 1608, 1544, 1497, 1402, 784 cm−1; 1H NMR (300 MHz, CDCl3): δ = 2.32 (s, 6H, CH3), 5.52 (s, 1H, CH), 7.33–7.42 (m, 17H, ArH) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 12.2, 31.4, 106.0, 120.5, 123.7, 125.4, 125.5, 126.1, 126.2, 127.4, 129.0, 129.1, 129.1, 131.2, 134.1, 137.0, 137.7, 146.1, 158.8 ppm; elemental analysis: requires: C, 76.52; H, 5.39; O, 6.58% (C31H26N4O2) found: C, 76.55; H, 5.32; O, 6.60%.
Acknowledgements
We gratefully acknowledged the financial support from the Department of Science Technology and University Grants Commission for FIST and SAP respectively. One of the authors Madhuri Barge thanks the Department of Science Technology, New Delhi, India for the award of PURSE fellow.References
- (a) P. Anastas and J. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998, p. 3 Search PubMed; (b) J. H. Clark, Green Chem., 1999, 1, 1 RSC.
- R. A. Sheldon, Green Chem., 2005, 7, 267 RSC.
- (a) S. Kobayashi, Pure Appl. Chem., 2007, 79, 235 CrossRef CAS; (b) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 CrossRef CAS; (c) R. Breslow, Acc. Chem. Res., 1991, 24, 159 CrossRef CAS.
- (a) S. Kobayashi and K. Manabe, Pure Appl. Chem., 2000, 72, 1373 CrossRef CAS; (b) S. Kobayashi and K. Manabe, Acc. Chem. Res., 2002, 35, 209 CrossRef CAS PubMed; (c) M. Shiri and M. A. Zolfigol, Tetrahedron, 2009, 65, 587 CrossRef CAS.
- (a) Y. Zhu, M. Durand, V. Molinier and J. M. Aubry, Green Chem., 2008, 10, 532 RSC; (b) S. Kamble, A. Kumbhar, M. Barge, G. Rashinkar and R. Salunkhe, Ultrason. Sonochem., 2012, 19, 812 CrossRef CAS.
- (a) S. Shirakawa, K. Liu, H. Ito and K. Maruoka, Chem. Commun., 2011, 47, 1515 RSC; (b) Y. Liu, B. A. Provencher, K. J. Bartelsona and L. Deng, Chem. Sci., 2011, 2, 1301 RSC.
- (a) F. He, P. Li, Y. Gu and G. Li, Green Chem., 2009, 11, 1768 Search PubMed; (b) M. Li, C. Chen, F. He and Y. Gu, Adv. Synth. Catal., 2010, 352, 519 CrossRef CAS; (c) D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493 RSC; (d) B. Zhou, J. Yang, M. Li and Y. Gu, Green Chem., 2011, 13, 2204 RSC; (e) Y. Gu and F. Jérôme, Chem. Soc. Rev., 2013, 42, 9550 RSC; (f) S. Sun, R. Bai and Y. Gu, Chem.–Eur. J., 2014, 20, 549 CrossRef CAS PubMed.
- R. S. Makkar and K. J. Rockne, Environ. Toxicol. Chem., 2003, 22, 2280 CrossRef CAS PubMed.
- (a) J. Desai and I. Banat, Microbiol. Mol. Biol. Rev., 1997, 61, 47 CAS; (b) A. Fiechter, Trends Biotechnol., 1992, 10, 208 CrossRef CAS PubMed; (c) W. Finnerty, Curr. Opin. Biotechnol., 1994, 5, 291 CrossRef CAS; (d) S. Lin, J. Chem. Technol. Biotechnol., 1996, 66, 109 CrossRef CAS; (e) S. Cameotra and R. Makkar, Appl. Microbiol. Biotechnol., 1998, 50, 520 CrossRef CAS PubMed.
- (a) R. Makkar and S. Cameotra, Appl. Microbiol. Biotechnol., 2002, 58, 428 CrossRef CAS PubMed; (b) S. Itoh, Fat Sci. Technol., 1987, 89, 470 Search PubMed; (c) M. Brown, Int. J. Cosmet. Sci., 1991, 13, 61 CrossRef CAS PubMed.
- V. Singh, P. Tripathi, J. R. Patel, M. L. Kori and V. K. Dixit, Int. J. Pharm. Clin. Res., 2009, 1, 40 Search PubMed.
- D. C. Jain, Phytochemistry, 1987, 26, 2223 CrossRef CAS.
- (a) A. Kumbhar, S. Kamble, M. Barge, G. Rashinkar and R. Salunkhe, Tetrahedron Lett., 2012, 53, 2756 CrossRef CAS; (b) M. Barge, S. Kamble, A. Kumbhar, G. Rashinkar and R. Salunkhe, Monatsh. Chem., 2013, 144, 1213 CrossRef CAS.
- (a) U. M. Shanmugavel, B. Kamaraj, P. Subbu, Y. Perumal and S. Dharmarajan, Bioorg. Med. Chem. Lett., 2010, 20, 7278 CrossRef PubMed; (b) B. F. Dai, L. P. Song, P. Y. Wang, H. Yi, W. G. Cao, G. F. Jin, S. Z. Zhu and M. Shao, Synlett, 2009, 1842 CAS; (c) A. R. Suresh Babu and R. Raghunathan, Synth. Commun., 2009, 39, 347 CrossRef CAS.
- M. Katarzyna, K. Piotr, P. Dariusz and M. Zbigniew, Croat. Chem. Acta, 2009, 82, 613 Search PubMed.
- H. N. Pati, D. Umashankar, D. C. Erik, J. Balzarini and R. Dimmock, J. Enzyme Inhib. Med. Chem., 2007, 22, 37 CrossRef CAS.
- J. L. Bullington, J. C. Cameron, J. E. Davis, J. H. Dodd, C. A. Harris, J. R. Henry, L. P. Gensey, K. C. Rupert and J. Siekierka, Bioorg. Med. Chem. Lett., 1998, 8, 2489 CrossRef CAS PubMed.
- R. Karthik, S. R. Jasmin, S. Sasikumar, S. B. Kalyan, A. J. M. Christina, A. Jagan and S. K. Sundara, Pharmacologyonline, 2008, 2, 176 Search PubMed.
- D. Y. Wu, Z. J. Ren, W. G. Cao and W. Q. Tong, Synth. Commun., 2005, 35, 3157 CrossRef CAS.
- P. H. Yang, Q. Z. Zhang and W. Sun, Res. Chem. Intermed., 2012, 38, 1063 CrossRef CAS.
- (a) H. J. Park, K. Lee, S. J. Park, B. Ahn, J. C. Lee, H. Y. Cho and K. I. Lee, Bioorg. Med. Chem. Lett., 2005, 15, 3307 CrossRef CAS PubMed; (b) M. P. Clark, S. K. Laughlin, M. J. Laufersweiler, R. G. Bookland, T. A. Brugel, A. Golebiowski, M. P. Sabat, J. A. Townes, J. C. VanRens, J. F. Djung, M. G. Natchus, B. De, L. C. Hsieh, S. C. Xu, R. L. Walter, M. J. Mekel, S. A. Heitmeyer, K. K. Brown, K. Juergens, Y. O. Taiwo and M. J. Janusz, J. Med. Chem., 2004, 11, 2724 CrossRef PubMed.
- I. H. Cho, J. Y. Noh, S. W. Park, H. C. Ryu, J. W. Lim, J. H. Kim, M. Y. Chae, D. H. Kim, S. H. Jung, H. J. Park, Y. H. Kim and I. K. Min, US Pat., 2, 004, 002, 532, 2004.
- M. P. Clark, S. K. Laughlin, A. Golebiowski, T. A. Brugel and M. Sabat, WO Patent, 2, 005, 047, 287, 2005.
- (a) H. Takeishi, Y. Kitatsuji, T. Kimura, Y. Meguro, Z. Yoshida and S. Kihara, Anal. Chim. Acta, 2001, 431, 69 CrossRef CAS; (b) S. A. Abdel-Latif, Synth. React. Inorg. Met.-Org. Chem., 2001, 31, 1355 CrossRef CAS.
- C. E. Rosiere and M. I. Grossman, Science, 1951, 113, 651 CAS.
- D. M. Bailey, P. E. Hansen, A. G. Hlavac, E. R. Baizman, J. Pearl, A. F. Defelice and M. E. Feigenson, J. Med. Chem., 1985, 28, 256 CrossRef CAS PubMed.
- R. N. Mahajan, F. H. Havaldar and P. S. Fernandes, J. Indian Chem. Soc., 1991, 68, 245 CAS.
- P. M. S. Chauhan, S. Singh and R. K. Chatterjee, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1993, 32, 858 Search PubMed.
- (a) D. Singh and D. Singh, J. Indian Chem. Soc., 1991, 68, 165 CAS; (b) M. Londershausen, Pestic. Sci., 1996, 48, 269 CrossRef CAS; (c) H. A. Lubs, The Chemistry of Synthetic Dyes and Pigments, American Chemical Society, Washington D. C., 1970 Search PubMed; (d) A. B. Uzoukwu, Polyhedron, 1993, 12, 2719 CrossRef CAS; (e) R. C. Maurya and R. Verma, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 1997, 36, 596 Search PubMed; (f) A. D. Garnovskii, A. I. Uraev and V. I. Minkin, ARKIVOC, 2004, 3, 29 Search PubMed.
- Y. J. Bai, J. Lu, Z. J. Wang, H. Y. Gan and H. R. Ma, Chin. J. Org. Chem., 2004, 6, 616 Search PubMed.
- D. Q. Shi, J. Chen, N. Wu, Q. Y. Zhuang and X. S. Wang, Chin. J. Org. Chem., 2005, 4, 405 Search PubMed.
- W. Wang, S. X. Wang, X. Y. Qin and J. T. Li, Synth. Commun., 2005, 35, 1263 CrossRef CAS.
- M. N. Elinson, A. S. Dorofeev, R. F. Nasybullin and G. I. Nikishin, Synthesis, 2008, 12, 1933 CrossRef.
- K. Sujatha, G. Shanthi, N. P. Selvam, S. Manoharan, P. T. Perumal and M. Rajendran, Bioorg. Med. Chem. Lett., 2009, 19, 4501 CrossRef CAS PubMed.
- K. Niknam, D. Saberi, M. Sadegheyan and A. Deris, Tetrahedron Lett., 2010, 51, 692 CrossRef CAS.
- E. Mosaddegh, A. Hassankhani and A. Baghizadehb, J. Chil. Chem. Soc., 2010, 55, 419 CrossRef CAS.
- K. Niknam and S. Mirzaee, Synth. Commun., 2011, 41, 2403 CrossRef CAS.
- M. Baghernejad and K. Niknam, Int. J. Chem., 2012, 4, 52 CAS.
- A. Khazaei, M. A. Zolfigol, A. R. M. Zare, Z. Asgari, M. Shekouhy, A. Zare and A. Hasaninejad, RSC Adv., 2012, 2, 8010 RSC.
- (a) L. Xiaoliu, D. Daming, W. Yongmei and M. Jiben, Sci. China, Ser. B: Chem., 1997, 40, 205 CrossRef; (b) L. Xiao, W. Yong, T. Bing, M. Teruo and M. Ji, J. Heterocycl. Chem., 1998, 35, 129 CrossRef.
| This journal is © The Royal Society of Chemistry 2014 |