Silicon-containing oligomeric poly(imido-amides) with amino moieties. Synthesis, characterization and thermal studies
Luis H. Tagle*,
Claudio A. Terraza,
Alain Tundidor-Camba and
Pablo A. Ortiz
Pontificia Universidad Católica de Chile, Organic Chemistry Department, P.O. Box 306, Santiago, Chile. E-mail: ltagle@uc.cl
First published on 19th June 2014
Abstract
Silicon-containing oligomeric poly(imido-amides) (PIAs) were synthesized from dicarboxylic imido-acids containing a Si atom, which were obtained from dianhydrides and the amino acids glycine, L-alanine, L-phenylalanine, L-valine, L-leucine, L-isoleucine and p-aminobenzoic acid (I–III-(a–g)), were polymerized with the diamine bis(4-aminophenyl)diphenylsilane. Monomeric dicarboxylic imido-acids and PIAs were characterized by IR and 1H, 13C and 29Si NMR spectroscopy and, when necessary, optical rotation, and the results were in agreement with the proposed structures. The yields were very good, greater than 90%, but the ηinh values were low, indicating that PIAs were of oligomeric nature, especially those derived from dicarboxylic imido-acids with aromatic groups bonded to the Si central atom. PIAs were soluble in polar aprotic solvents, and also in other organic solvents like m-cresol, tetrahydrofuran, and several in CHCl3 and acetone, due to the inclusion of Si atoms and amino acidic residues in the main chain. The glass transition temperature (Tg) values were obtained by DSC, and showed a tendency in the sense that when the side chain of the amino acidic residue is increased, the Tg values decrease, as the higher volume of the side chain implies a higher chain separation and consequently a higher free rotation of them. PIAs including glycine or p-aminobenzoic acid residues, without side groups, showed higher Tg values due to an increase of the molecular rigidity. The thermal stability was determined by dynamic thermogravimetry showing that almost all PIAs were thermally stable, with TDT10% values greater than 400 °C. The most stable PIAs were those including the p-aminobenzoic residue in the main chain. The groups, methyl or phenyl, bonded to the Si atom of the carboxylic imide-acids residue did not show an important influence. The UV-vis transparency was studied showing that the increase of the aromatic content decreases the UV-vis transparency. PIAs derived from carboxylic imide-acids containing only phenyl groups bonded to the Si atom, were non-transparent.
Introduction
New materials are characterized by specific criteria, such as high thermal and mechanical resistance, low specific density, high conductivity and good electric properties.1Poly(amides), poly(imides) and other kinds of condensation polymers with aromatic content are high performance materials. Their excellent thermal and mechanical properties are very useful for use in advanced technologies. Poly(p-phenylene-terephthalime) and poly(m-phenylene-isophthalamide) are examples of materials used in aeroespatial industries as electrical insulators among other applications.
One of the main problems of condensation polymers like aromatic poly(amides) and poly(imides) is the low solubility which affects their processability. The solubility can be improved including flexible or polar groups in the polymeric chain. If these groups are correctly chosen it is possible to increase the solubility without affect the mechanical and thermal properties. The changes are based in to reduce the symmetry and the regularity of the system, in order to reduce the molecular interactions associated to the low solubility and high glass transition temperatures.
The introduction of flexible units in the polymeric chain,2 the use of meta-oriented units,3,4 the presence of non-planar units,5 the inclusion of bulky groups6–10 and the presence of two or more functional groups in the polymeric chain,11,12 can to produce soluble polymers with moderate Tg values but maintaining the thermal properties.
One of the most recent methods for improve the properties is the inclusion of stereogenic centers from chiral compounds, usually from natural α-amino acids, in order to obtain more soluble polymers with a higher specific regularity.13–15 Mallakpour et al. have described several polymers containing chiral centers in their structure including L-α-amino acids in the main chain.16–20
On the other hand, the introduction of silicon atoms in the main chain implies an electronic transport due to the interaction between the π orbitals of the aromatic rings and the d orbital of the heteroatom. Also with this heteroatom in the polymeric chain, the solubility and the thermal stability can increase due to the higher ionic character of the Si–C bond as a consequence of the difference of electronegativity between the atoms.21
After the first Si-containing condensation polymer was reported by Speck,22 a great number of this kind of polymers were described by several authors,21–32 showing the importance of this kind of polymers including Si in the main chain, which have shown several technological uses like membranes, insulators films and potential optoelectronic materials.33,34
Also the synthesis of Si-containing polymers including two functional groups like poly(imide-amides) or poly(imide-ethers) have been described as examples of materials with flexible units which improve the solubility maintaining the thermal resistance.24
Continuing our works about of Si-containing condensation polymers,35–42 we describe the synthesis of poly(imide-amides) containing two Si atoms and both functional groups, imide and amide. In fact, dianhydrides with a Si atom in their structure reacted with several natural L-amino acids and p-aminobenzoic acid in order to obtain monomeric dicarboxylic acids with the imide group and a flexible aliphatic residue provided by the amino acid, which were polymerized with an aromatic diamine containing another Si atom as central element. Poly(imide-amides) were structurally characterized by spectroscopic methods and the thermal properties determined and related with the groups bonded to the Si atom of the dicarboxylic imido-acids and the aminoacidic structure.
Experimental part
Materials
Glycine, L-alanine, L-phenylalanine, L-valine, L-leucine, L-isoleucine, p-amino-benzoic acid and dimethyldichlorosilane, phenylmethyldichlorosilane, and diphenyldichlorosilane were obtained from Aldrich Chemical (Milwaukee, WI) and used without further purification. The 4-bromo-o-xylene was obtained from AlphaAesar with 98% of purity. All other reagents and solvents were purchased commercially as analytical-grade and used without further purification.Instrumentation
IR spectra (KBr pellets) were recorded on a Perkin-Elmer (Fremont CA) 1310 spectrophotometer over the range of 450–4000 cm−1.1H, 13C and 29Si NMR spectra were carried out on a 400 MHz instrument (Bruker AC-200) using DMSO-d6, CDCl3 or acetone-d6 as solvents and TMS as the internal standard. Viscosimetric measurements were made in a Desreux-Bischof type dilution viscosimeter at 25 °C (c = 0.5 g dL−1). Tg values were obtained with a Mettler-Toledo (Greifensee, Switzerland) DSC 821 calorimetric system (20 °C min−1 under N2 flow) after the second heating run. Thermogravimetric analyses were carried out in a Mettler (Switzerland) TA-3000 calorimetric system equipped with a TC-10A processor, and a TG-50 thermobalance with a Mettler MT5 microbalance. Samples of 6–10 mg were placed in a platinum sample holder and the thermogravimetric measurements were carried out between 30 °C and 800 °C with a heating rate of 20 °C min−1 under N2 flow. Specific rotations were measured in an Optical Activity Automatic polarimeter, Model AA-5 at 25 °C. The UV-visible optical transmission spectra were obtained in NMP solutions (c = 5.0 g L−1) at room temperature on a UV-3101PC UV-Vis-NIR scanning spectrophotometer (Shimadzu, Japan).Monomers
Tetramethyl derivatives (TM (I–III)) were obtained from the lithiated derivative of 4-bromo-o-xylene and the dichlorodialkyl- or diaryl-silanes in diethyl ether, and then oxidized to the tetracarboxylic imido-acids (TA (I–III)) with KMnO4 and pyridine–water mixture according to described procedures. The tetracarboxylic imido-acids were cyclized to the dianhydrides (DA (I–III)) with acetic anhydride–acetic acid mixture according to a reported procedure (Scheme 1).43–47Twenty one monomeric dicarboxylic imido-acids ((I–III) (a–g)) were obtained by the reaction between the dianhydrides and the mentioned amino acids in acetic acid.48–51 In a typical reaction, 0.01 mmol of the dianhydride and 0.025 mmol of the amino acid were mixed in 30 mL of acetic acid and stirred by four hours and then, refluxed other four hours. After this time the acetic acid was destilled under reduce pressure and the residue was poured into a 10% of aqueous HCl solution. The solid was filtered, washed, dried under vacuum and structurally characterized.
Series I (R1 = R2 = Me)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1603, 1480 (C
O), 1603, 1480 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1421, 1390 (C–H aliph.), 1225 (Si–CH3), 1120 (Si–C6H5), 878, 833 (arom. 1,2,4-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.82 (s,6H,11), 4.44 (s,4H,9), 7.90 (d,J = 7.3 Hz,2H,6), 8.10 (s,2H,2), 8.12 (d,J = 7.3 Hz,4H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.0 (11), 39.2 (9), 123.3 (5), 129.3 (2), 132.1 (3), 133.8 (4), 141.2 (6), 146.7 (1), 168.0, 168.2 (8,7), 168.9 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.47.
C arom.), 1421, 1390 (C–H aliph.), 1225 (Si–CH3), 1120 (Si–C6H5), 878, 833 (arom. 1,2,4-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.82 (s,6H,11), 4.44 (s,4H,9), 7.90 (d,J = 7.3 Hz,2H,6), 8.10 (s,2H,2), 8.12 (d,J = 7.3 Hz,4H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.0 (11), 39.2 (9), 123.3 (5), 129.3 (2), 132.1 (3), 133.8 (4), 141.2 (6), 146.7 (1), 168.0, 168.2 (8,7), 168.9 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.47.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1612, 1564 (C
O), 1612, 1564 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1450, 1383 (C–H aliph.), 1414, 1153 (Si–C6H5), 1252 (Si–CH3), 836, 8913 (arom. 1,2,4-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.82 (s,6H,11), 1.68 (d,J = 7.3 Hz,4H,16), 4.99 (q,J = 7.4 Hz,2H,9), 7.89 (d,J = 7.4 Hz,2H,6), 8.07 (s,2H,2), 8.11 (d,J = 7.4 Hz,4H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.0 (11), 15.3 (16), 47.9 (9), 123.4 (5), 129.2 (2), 132.1 (3), 133.7 (4), 141.1 (6), 146.6 (1), 168.0, 168.2 (8,7), 171.2 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.47.
C arom.), 1450, 1383 (C–H aliph.), 1414, 1153 (Si–C6H5), 1252 (Si–CH3), 836, 8913 (arom. 1,2,4-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.82 (s,6H,11), 1.68 (d,J = 7.3 Hz,4H,16), 4.99 (q,J = 7.4 Hz,2H,9), 7.89 (d,J = 7.4 Hz,2H,6), 8.07 (s,2H,2), 8.11 (d,J = 7.4 Hz,4H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.0 (11), 15.3 (16), 47.9 (9), 123.4 (5), 129.2 (2), 132.1 (3), 133.7 (4), 141.1 (6), 146.6 (1), 168.0, 168.2 (8,7), 171.2 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.47.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1606 1497 (C
O), 1606 1497 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1455, 1379 (C–H aliph.), 1413, 1104 (Si–C6H5), 1251 (Si–CH3), 872, 835 (arom. 1,2,4-subst.) 741, 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.75 (s,6H,11), 3.56 (m,4H,17), 5.21 (dd,J = 11.5,5.0 Hz,2H,9), 7.21 (m,10H,19,20,21), 7.79 (d,J = 7.4 Hz,2H,6), 7.97 (s,2H,2), 8.03 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.1 (11), 35.1 (17), 54.0 (9), 123.2 (5), 127.5 (21), 129.2 (2), 129.3 (19), 129.7 (20), 131.6 (3), 133.3 (4), 138.4 (18), 141.3 (6), 146.7 (1), 168.1, 168.3 (7,8), 170.3 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.52.
C), 1455, 1379 (C–H aliph.), 1413, 1104 (Si–C6H5), 1251 (Si–CH3), 872, 835 (arom. 1,2,4-subst.) 741, 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.75 (s,6H,11), 3.56 (m,4H,17), 5.21 (dd,J = 11.5,5.0 Hz,2H,9), 7.21 (m,10H,19,20,21), 7.79 (d,J = 7.4 Hz,2H,6), 7.97 (s,2H,2), 8.03 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.1 (11), 35.1 (17), 54.0 (9), 123.2 (5), 127.5 (21), 129.2 (2), 129.3 (19), 129.7 (20), 131.6 (3), 133.3 (4), 138.4 (18), 141.3 (6), 146.7 (1), 168.1, 168.3 (7,8), 170.3 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.52.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1610, 1546 (C
O). 1610, 1546 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1466 (C–H aliph.), 1414, 1121 (Si–C6H5), 1269 (Si–CH3) 887, 834 (arom. 1,2,4-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.83 (s,6H,11), 0.90 (d,J = 6.6 Hz,6H,23), 1.16 (d,J = 6.8 Hz,6H,24), 2.71 (m,2H,22), 4.57 (d,J = 8.1 Hz,2H,9), 7.91 (d,J = 7.4 Hz,2H,6), 8.11 (s,4H,2) 8.14 (d,J = 7.4 Hz,4H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.0 (11), 19.8 (22), 21.3 (23), 29.2 (24), 57.9 (9), 123.4 (5), 129.3 (2), 131.8 (3), 133.5 (4), 141.3 (6), 146.8 (1), 168.4, 168.6 (7,8), 170.0 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.50.
C), 1466 (C–H aliph.), 1414, 1121 (Si–C6H5), 1269 (Si–CH3) 887, 834 (arom. 1,2,4-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.83 (s,6H,11), 0.90 (d,J = 6.6 Hz,6H,23), 1.16 (d,J = 6.8 Hz,6H,24), 2.71 (m,2H,22), 4.57 (d,J = 8.1 Hz,2H,9), 7.91 (d,J = 7.4 Hz,2H,6), 8.11 (s,4H,2) 8.14 (d,J = 7.4 Hz,4H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.0 (11), 19.8 (22), 21.3 (23), 29.2 (24), 57.9 (9), 123.4 (5), 129.3 (2), 131.8 (3), 133.5 (4), 141.3 (6), 146.8 (1), 168.4, 168.6 (7,8), 170.0 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.50.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1610, 1546 (C
O), 1610, 1546 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1469, 1379 (C–H aliph.), 1414, 1126 (Si–C6H5), 1267 (Si–CH3), 859, 836 (arom. 1,2,4-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.83 (s,6H,11), 0.92 (d,J = 6.7 Hz,6H,26), 0.95 (d,J = 6.5 Hz,6H,27), 1.54 (m,2H,25), 1.96 (m,2H,17), 2.34 (m,2H,17′), 4.95 (dd,J = 11.6,4.3 Hz,2H,9), 7.91 (d,J = 7.4 Hz,2H,6), 8.10 (s,2H,2), 8.13 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.0 (11), 21.2 (26), 23.5 (27), 25.7 (25), 37.9 (17), 51.0 (9), 123.3 (5), 129.3 (2), 131.9 (3), 133.6 (4), 141.2 (6), 146.7 (1), 168.3, 168.6 (7,8), 171.1 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.50.
C arom.), 1469, 1379 (C–H aliph.), 1414, 1126 (Si–C6H5), 1267 (Si–CH3), 859, 836 (arom. 1,2,4-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.83 (s,6H,11), 0.92 (d,J = 6.7 Hz,6H,26), 0.95 (d,J = 6.5 Hz,6H,27), 1.54 (m,2H,25), 1.96 (m,2H,17), 2.34 (m,2H,17′), 4.95 (dd,J = 11.6,4.3 Hz,2H,9), 7.91 (d,J = 7.4 Hz,2H,6), 8.10 (s,2H,2), 8.13 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.0 (11), 21.2 (26), 23.5 (27), 25.7 (25), 37.9 (17), 51.0 (9), 123.3 (5), 129.3 (2), 131.9 (3), 133.6 (4), 141.2 (6), 146.7 (1), 168.3, 168.6 (7,8), 171.1 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.50.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1610, 1545 (C
O), 1610, 1545 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1461, 1375 (C–H aliph.), 1266 (Si–CH3), 1413, 1125 (Si–C6H5), 874, 835 (arom. 1,2,4-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.82 (s,6H,11), 0.85 (t,J = 7.5 Hz,6H,29), 1.10 (m,2H,28), 1.14 (d,J = 6.7 Hz,6H,23), 1.54 (m,2H,28′), 2.51 (m,2H,22), 4.62 (d,J = 8.2 Hz,2H,9), 7.90 (d,J = 7.4 Hz,2H,6), 8.10 (s,2H,2), 8.13 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.0 (11), 11.2 (29), 17.3 (23), 26.5 (28), 35.2 (22), 57.4 (9), 123.3 (5), 129.3 (2), 131.8 (3), 133.5 (4), 141.3 (6), 146.8 (1), 168.4, 168.6 (7,8), 170.3 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.50.
C arom.), 1461, 1375 (C–H aliph.), 1266 (Si–CH3), 1413, 1125 (Si–C6H5), 874, 835 (arom. 1,2,4-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.82 (s,6H,11), 0.85 (t,J = 7.5 Hz,6H,29), 1.10 (m,2H,28), 1.14 (d,J = 6.7 Hz,6H,23), 1.54 (m,2H,28′), 2.51 (m,2H,22), 4.62 (d,J = 8.2 Hz,2H,9), 7.90 (d,J = 7.4 Hz,2H,6), 8.10 (s,2H,2), 8.13 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.0 (11), 11.2 (29), 17.3 (23), 26.5 (28), 35.2 (22), 57.4 (9), 123.3 (5), 129.3 (2), 131.8 (3), 133.5 (4), 141.3 (6), 146.8 (1), 168.4, 168.6 (7,8), 170.3 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.50.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1609, 1582, 1514 (C
O), 1609, 1582, 1514 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1426, 1374 (C–H aliph.), 1265 (Si–CH3); 1125, 1411 (Si–C6H5), 857, 830 (arom. 1,2,4-subst.), 813 (arom. p-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.79 (s,6H,11), 7.61 (d,J = 8.5 Hz,4H,31), 8.00 (d,J = 7.4 Hz,2H,6), 8.13 (m,8H,2,5,20), 13.13 (s,2H,OH). 13C NMR (acetone-d6) (δ) (ppm): −3.41 (11), 122.7 (5), 126.9 (31), 128.4 (2), 129.8 (32), 129.9 (33), 130.7 (3), 132.4 (4), 135.7 (30), 140.3 (6), 145.7 (1), 166.5, 166.7 (7,8), 166.8 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.51.
C arom.), 1426, 1374 (C–H aliph.), 1265 (Si–CH3); 1125, 1411 (Si–C6H5), 857, 830 (arom. 1,2,4-subst.), 813 (arom. p-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.79 (s,6H,11), 7.61 (d,J = 8.5 Hz,4H,31), 8.00 (d,J = 7.4 Hz,2H,6), 8.13 (m,8H,2,5,20), 13.13 (s,2H,OH). 13C NMR (acetone-d6) (δ) (ppm): −3.41 (11), 122.7 (5), 126.9 (31), 128.4 (2), 129.8 (32), 129.9 (33), 130.7 (3), 132.4 (4), 135.7 (30), 140.3 (6), 145.7 (1), 166.5, 166.7 (7,8), 166.8 (10). 29Si NMR (acetone-d6) (δ) (ppm): −4.51.Series II (R1 = Me; R2 = C6H5)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1614 (C
O), 1614 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1423, 1391 (C–H aliph.), 879, 851 (arom. 1,2,4-subst.), 749, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 1.11 (s,3H,11), 4.43 (s,4H,9), 7.48 (m,3H,14,15), 7.63 (d,J = 7.7 Hz,2H,13), 7.94 (d,J = 7.4 Hz,2H,6), 8.05 (s,2H,2), 8.08 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.9 (11), 32.9 (9), 123.5 (5), 129.3 (14), 130.1 (15), 131.7 (2), 132.2 (3), 133.9 (12), 134.1 (4), 136.0 (13), 142.2 (6), 144.7 (1), 167.9, 168.1 (7,8), 168.8 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.76.
C arom.), 1423, 1391 (C–H aliph.), 879, 851 (arom. 1,2,4-subst.), 749, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 1.11 (s,3H,11), 4.43 (s,4H,9), 7.48 (m,3H,14,15), 7.63 (d,J = 7.7 Hz,2H,13), 7.94 (d,J = 7.4 Hz,2H,6), 8.05 (s,2H,2), 8.08 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.9 (11), 32.9 (9), 123.5 (5), 129.3 (14), 130.1 (15), 131.7 (2), 132.2 (3), 133.9 (12), 134.1 (4), 136.0 (13), 142.2 (6), 144.7 (1), 167.9, 168.1 (7,8), 168.8 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.76.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1610, 1545 (C
O), 1610, 1545 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1451, 1382 (C–H aliph.), 849, 830 (arom. 1,2,4-subst.), 741, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 1.11 (s,3H,11), 1.68 (d,J = 7.3 Hz,6H,16), 4.99 (q,J = 7.3 Hz,2H,9), 7.49 (m,3H,14,15), 7.63 (d,J = 6.5 Hz,2H,13), 7.92 (d,J = 7.4 Hz,2H,6), 8.01 (s,2H,2), 8.07 (d,J = 7.3 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.9 (11), 15.3 (16), 48.0 (9), 123.4 (5), 129.3 (14), 130.0 (15), 131.3 (2), 132.1 (3), 134.0 (12), 134.1 (4), 136.0 (13), 142.1 (6), 144.6 (1), 167.9, 168.1 (7,8), 171.2 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.85.
C arom.), 1451, 1382 (C–H aliph.), 849, 830 (arom. 1,2,4-subst.), 741, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 1.11 (s,3H,11), 1.68 (d,J = 7.3 Hz,6H,16), 4.99 (q,J = 7.3 Hz,2H,9), 7.49 (m,3H,14,15), 7.63 (d,J = 6.5 Hz,2H,13), 7.92 (d,J = 7.4 Hz,2H,6), 8.01 (s,2H,2), 8.07 (d,J = 7.3 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.9 (11), 15.3 (16), 48.0 (9), 123.4 (5), 129.3 (14), 130.0 (15), 131.3 (2), 132.1 (3), 134.0 (12), 134.1 (4), 136.0 (13), 142.1 (6), 144.6 (1), 167.9, 168.1 (7,8), 171.2 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.85.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1607, 1547, 1497 (C
O), 1607, 1547, 1497 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1455 (C–H aliph.), 1265 (Si–CH3), 1414, 1107 (Si–C6H5), 871, 850 (arom. 1,2,4-subst.), 740, 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 1.05 (s,3H,11), 3.57 (m,4H,17), 5.24 (dd,J = 11.4,5.0 Hz,2H,9), 7.20 (m,10H,19,20,20), 7.50 (m,5H,13,14,15), 7.83 (d,J = 7.3 Hz,2H,6), 7.91 (s,2H,2), 7.99 (d,J = 7.3 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.98 (11), 35.1 (17), 53.9 (9), 123.4 (8), 127.5 (21), 129.2 (19), 129.3 (2), 129.7 (20), 130.0 (14), 131.3 (15), 131.7 (3), 133.6 (4), 133.9 (12), 136.0 (13), 138.3 (18), 142.3 (6), 144.7 (1), 168.0, 168.1 (7,8), 170.3 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.93.
C arom.), 1455 (C–H aliph.), 1265 (Si–CH3), 1414, 1107 (Si–C6H5), 871, 850 (arom. 1,2,4-subst.), 740, 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 1.05 (s,3H,11), 3.57 (m,4H,17), 5.24 (dd,J = 11.4,5.0 Hz,2H,9), 7.20 (m,10H,19,20,20), 7.50 (m,5H,13,14,15), 7.83 (d,J = 7.3 Hz,2H,6), 7.91 (s,2H,2), 7.99 (d,J = 7.3 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.98 (11), 35.1 (17), 53.9 (9), 123.4 (8), 127.5 (21), 129.2 (19), 129.3 (2), 129.7 (20), 130.0 (14), 131.3 (15), 131.7 (3), 133.6 (4), 133.9 (12), 136.0 (13), 138.3 (18), 142.3 (6), 144.7 (1), 168.0, 168.1 (7,8), 170.3 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.93.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1611, 1543 (C
O), 1611, 1543 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1466, 1378 (C–H aliph.), 1269 (Si–CH3); 1414, 1110 (Si–C6H5); 848, 828 (arom. 1,2,4-subst.), 742, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.90 (d,6H,23), 1.12 (11), 1.16 (d,6H,24), 2.71 (m,2H,22), 4.58 (d,J = 8.0 Hz,2H,9), 7.49 (m,3H,14,15), 7.64 (d,2H,13), 7.95 (d,J = 7.4 Hz,2H,6), 8.04 (s,2H,2), 8.10 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.84 (11), 19.8 (23), 21.3 (24), 29.2 (22), 58.0 (9), 123.5 (5), 129.3 (14), 130.2 (15), 131.3 (2), 131.9 (3), 133.8 (12), 134.0 (4), 136.1 (13), 142.3 (6), 144.8 (1), 168.3, 168.5 (7,8), 170.0 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.86.
C arom.), 1466, 1378 (C–H aliph.), 1269 (Si–CH3); 1414, 1110 (Si–C6H5); 848, 828 (arom. 1,2,4-subst.), 742, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.90 (d,6H,23), 1.12 (11), 1.16 (d,6H,24), 2.71 (m,2H,22), 4.58 (d,J = 8.0 Hz,2H,9), 7.49 (m,3H,14,15), 7.64 (d,2H,13), 7.95 (d,J = 7.4 Hz,2H,6), 8.04 (s,2H,2), 8.10 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.84 (11), 19.8 (23), 21.3 (24), 29.2 (22), 58.0 (9), 123.5 (5), 129.3 (14), 130.2 (15), 131.3 (2), 131.9 (3), 133.8 (12), 134.0 (4), 136.1 (13), 142.3 (6), 144.8 (1), 168.3, 168.5 (7,8), 170.0 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.86.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1610 (C
O), 1610 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1469, 1414, 1380 (C–H aliph.), 1266 (Si–CH3); 1414, 1157 (Si–C6H5), 859 (arom. 1,2,4-subst.), 741 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.91 (d,6H,26), 0.95 (d,6H,27), 1.12 (s,3H,11), 1.54 (m,2H,25), 1.97 (t,2H,17), 2.33 (t,2H,17′), 4.95 (dd,J = 11.5,4.1 Hz,2H,9), 7.48 (m,3H,14,15), 7.64 (d,2H,13), 7.94 (d,J = 7.4 Hz,2H,6), 8.03 (s,2H,2), 8.09 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.8 (11), 21.3 (26), 23.5 (27), 25.8 (25), 38.0 (17), 51.1 (9), 123.5 (5), 129.4 (14), 130.2 (15), 131.3 (2), 132.0 (3), 133.9 (12), 134.0 (4), 136.1 (13), 142.3 (6), 144.8 (1), 168.3, 168.5 (7,8), 171.1 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.84.
C arom.), 1469, 1414, 1380 (C–H aliph.), 1266 (Si–CH3); 1414, 1157 (Si–C6H5), 859 (arom. 1,2,4-subst.), 741 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.91 (d,6H,26), 0.95 (d,6H,27), 1.12 (s,3H,11), 1.54 (m,2H,25), 1.97 (t,2H,17), 2.33 (t,2H,17′), 4.95 (dd,J = 11.5,4.1 Hz,2H,9), 7.48 (m,3H,14,15), 7.64 (d,2H,13), 7.94 (d,J = 7.4 Hz,2H,6), 8.03 (s,2H,2), 8.09 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.8 (11), 21.3 (26), 23.5 (27), 25.8 (25), 38.0 (17), 51.1 (9), 123.5 (5), 129.4 (14), 130.2 (15), 131.3 (2), 132.0 (3), 133.9 (12), 134.0 (4), 136.1 (13), 142.3 (6), 144.8 (1), 168.3, 168.5 (7,8), 171.1 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.84.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1611, 1543 (C
O), 1611, 1543 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1461, 1376 (C–H aliph.), 1264 (Si–CH3), 1414, 1109 (Si–C6H5), 872, 840 (arom. 1,2,4-subst), 741, 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.85 (t,6H,29), 1.07 (m,2H,28), 1.12 (s,3H,11), 1.14 (d,6H,23), 1.55 (m,2H,28′), 2.49 (m,2H,22), 4.64 (d,2H,9), 7.48 (d,3H,14,15), 7.64 (d,2H,13), 7.94 (d,J = 7.4 Hz,2H,6), 8.04 (s,2H,2), 8.09 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.9 (11), 11.3 (29), 17.3 (23), 26.5 (28), 35.2 (22), 57.4 (9), 123.5 (5), 129.3 (14), 130.2 (15), 131.3 (2), 131.9 (3), 133.8 (12), 134.0 (4), 136.1 (13), 142.3 (6), 144.8 (1), 168.3, 168.5 (7,8), 170.1 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.85.
C arom.), 1461, 1376 (C–H aliph.), 1264 (Si–CH3), 1414, 1109 (Si–C6H5), 872, 840 (arom. 1,2,4-subst), 741, 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.85 (t,6H,29), 1.07 (m,2H,28), 1.12 (s,3H,11), 1.14 (d,6H,23), 1.55 (m,2H,28′), 2.49 (m,2H,22), 4.64 (d,2H,9), 7.48 (d,3H,14,15), 7.64 (d,2H,13), 7.94 (d,J = 7.4 Hz,2H,6), 8.04 (s,2H,2), 8.09 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): −3.9 (11), 11.3 (29), 17.3 (23), 26.5 (28), 35.2 (22), 57.4 (9), 123.5 (5), 129.3 (14), 130.2 (15), 131.3 (2), 131.9 (3), 133.8 (12), 134.0 (4), 136.1 (13), 142.3 (6), 144.8 (1), 168.3, 168.5 (7,8), 170.1 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.85.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1608, 1579, 1543, 1513 (C
O), 1608, 1579, 1543, 1513 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1475, 1354 (C–H aliph.), 1288 (Si–CH3), 1425, 1114 (Si–C6H5), 854 (arom. p-subst.), 737, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 1.10 (s,3H,11), 7.55 (m,9H,13,14,15,30), 8.07 (m,10H,2,5,6,32), 13.14 (s,2H,OH). 13C NMR (acetone-d6) (δ) (ppm): −4.44 (11), 123.0 (5), 126.9 (31), 128.4 (14), 129.0 (15), 129.8 (32), 130.0 (33), 130.4 (2), 130.9 (3), 132.8 (4), 132.9 (12), 134.9 (13), 135.7 (30), 141.2 (6), 143.4 (1), 166.5, 166.5 (7,8), 166.7 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.80.
C arom.), 1475, 1354 (C–H aliph.), 1288 (Si–CH3), 1425, 1114 (Si–C6H5), 854 (arom. p-subst.), 737, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 1.10 (s,3H,11), 7.55 (m,9H,13,14,15,30), 8.07 (m,10H,2,5,6,32), 13.14 (s,2H,OH). 13C NMR (acetone-d6) (δ) (ppm): −4.44 (11), 123.0 (5), 126.9 (31), 128.4 (14), 129.0 (15), 129.8 (32), 130.0 (33), 130.4 (2), 130.9 (3), 132.8 (4), 132.9 (12), 134.9 (13), 135.7 (30), 141.2 (6), 143.4 (1), 166.5, 166.5 (7,8), 166.7 (10). 29Si NMR (acetone-d6) (δ) (ppm): −8.80.Series III (R1 = R2 = C6H5)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1611 (C
O), 1611 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1485 (C–H aliph.), 1425, 1116 (Si–C6H5), 878, 851 (arom. 1,2,4-subst.), 731, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 4.44 (s,4H,9), 7.59 (m,10H,13,14,15), 8.00 (d,J = 6.6 Hz,2H,6), 8.05 (s,2H,2), 8.13 (d, J = 6.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): 39.3. (9), 123.7 (5), 129.5 (14), 130.9 (15), 131.6 (2), 132.1 (3), 132.3 (4), 134.4 (12), 137.1 (13), 142.6 (6), 143.3 (1), 167.9, 168.0 (7,8), 168.9 (10). 29Si NMR (acetone-d6) (δ) (ppm): −13.45.
C arom.), 1485 (C–H aliph.), 1425, 1116 (Si–C6H5), 878, 851 (arom. 1,2,4-subst.), 731, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 4.44 (s,4H,9), 7.59 (m,10H,13,14,15), 8.00 (d,J = 6.6 Hz,2H,6), 8.05 (s,2H,2), 8.13 (d, J = 6.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): 39.3. (9), 123.7 (5), 129.5 (14), 130.9 (15), 131.6 (2), 132.1 (3), 132.3 (4), 134.4 (12), 137.1 (13), 142.6 (6), 143.3 (1), 167.9, 168.0 (7,8), 168.9 (10). 29Si NMR (acetone-d6) (δ) (ppm): −13.45.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1458, 1383 (C–H aliph.), 1415, 1108 (Si–C6H5), 849 (arom. 1,2,4-subst.), 744, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 1.68 (d,J = 7.3 Hz,6H,16), 5.00 (q,J = 7.2 Hz,2H,9), 7.54 (m,6H,14,15), 7.66 (d,J = 6.8 Hz,4H,13), 7.98 (d,J = 7.4 Hz,2H,6), 8.02 (s,2H,2), 8.12 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): 15.3 (16), 48.0 (9), 123.6 (5), 129.5 (14), 130.9 (15), 131.6 (2), 132.2 (3), 132.3 (4), 134.3 (12), 137.1 (13), 142.6 (6), 143.2 (1), 167.8, 168.0 (7,8), 171.1 (10). 29Si NMR (acetone-d6) (δ) (ppm): −13.53.
O), 1458, 1383 (C–H aliph.), 1415, 1108 (Si–C6H5), 849 (arom. 1,2,4-subst.), 744, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 1.68 (d,J = 7.3 Hz,6H,16), 5.00 (q,J = 7.2 Hz,2H,9), 7.54 (m,6H,14,15), 7.66 (d,J = 6.8 Hz,4H,13), 7.98 (d,J = 7.4 Hz,2H,6), 8.02 (s,2H,2), 8.12 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): 15.3 (16), 48.0 (9), 123.6 (5), 129.5 (14), 130.9 (15), 131.6 (2), 132.2 (3), 132.3 (4), 134.3 (12), 137.1 (13), 142.6 (6), 143.2 (1), 167.8, 168.0 (7,8), 171.1 (10). 29Si NMR (acetone-d6) (δ) (ppm): −13.53.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1608, 1543, 1497 (C
O), 1608, 1543, 1497 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1455 (C–H aliph.), 1414, 1106 (Si–C6H5), 870, 848 (arom. 1,2,4-subst.), 742, 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 3.57 (m,4H,17), 5.25 (dd,J = 11.2,4.8 Hz,2H,9), 7.17 (m,10H,19,20,21), 7.53 (m,10H,13,14,15), 7.89 (d,J = 7.5 Hz,2H,6), 7.91 (s,2H,2), 8.05 (d,J = 7.5 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): 35.1 (17), 54.0 (9), 123.5 (5), 127.5 (21), 129.3 (13), 129.4 (19), 129.7 (20), 130.8 (15), 131.6 (2), 131.7 (3), 131.9 (4), 133.8 (12), 137.1 (14), 138.3 (18), 142.6 (6), 143.3 (1), 167.9, 168.0 (7,8), 170.2 (10). 29Si NMR (acetone-d6) (δ) (ppm): −13.66.
C arom.), 1455 (C–H aliph.), 1414, 1106 (Si–C6H5), 870, 848 (arom. 1,2,4-subst.), 742, 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 3.57 (m,4H,17), 5.25 (dd,J = 11.2,4.8 Hz,2H,9), 7.17 (m,10H,19,20,21), 7.53 (m,10H,13,14,15), 7.89 (d,J = 7.5 Hz,2H,6), 7.91 (s,2H,2), 8.05 (d,J = 7.5 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): 35.1 (17), 54.0 (9), 123.5 (5), 127.5 (21), 129.3 (13), 129.4 (19), 129.7 (20), 130.8 (15), 131.6 (2), 131.7 (3), 131.9 (4), 133.8 (12), 137.1 (14), 138.3 (18), 142.6 (6), 143.3 (1), 167.9, 168.0 (7,8), 170.2 (10). 29Si NMR (acetone-d6) (δ) (ppm): −13.66.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1611, 1546 (C
O), 1611, 1546 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1467, 1428 (C–H aliph.), 1414, 1109 (Si–C6H5), 848, 828 (arom. 1,2,4.subst.), 728, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.91 (d,J = 6.8 Hz,6H,23), 1.15 (d,J = 6.7 Hz,6H,24), 2.70 (m,2H,22), 4.58 (d, J = 8.0 Hz,2H,9), 7.53 (m,6H,14,15), 7.66 (d,4H,13), 8.00 (d,J = 7.4 Hz,2H,6), 8.04 (s,2H,2), 8.14 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): 19.8 (23), 21.3 (24), 29.2 (22), 58.0 (9), 123.7 (5), 129.5 (14), 131.0 (15), 131.6 (2), 132.0 (3), 132.1 (4), 134.1 (12), 137.1 (13), 142.7 (6), 143.3 (1), 168.3, 168.4 (7,8), 170.1 (10). 29Si NMR (acetone-d6) (δ) (ppm): −13.55.
C arom.), 1467, 1428 (C–H aliph.), 1414, 1109 (Si–C6H5), 848, 828 (arom. 1,2,4.subst.), 728, 701 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.91 (d,J = 6.8 Hz,6H,23), 1.15 (d,J = 6.7 Hz,6H,24), 2.70 (m,2H,22), 4.58 (d, J = 8.0 Hz,2H,9), 7.53 (m,6H,14,15), 7.66 (d,4H,13), 8.00 (d,J = 7.4 Hz,2H,6), 8.04 (s,2H,2), 8.14 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): 19.8 (23), 21.3 (24), 29.2 (22), 58.0 (9), 123.7 (5), 129.5 (14), 131.0 (15), 131.6 (2), 132.0 (3), 132.1 (4), 134.1 (12), 137.1 (13), 142.7 (6), 143.3 (1), 168.3, 168.4 (7,8), 170.1 (10). 29Si NMR (acetone-d6) (δ) (ppm): −13.55.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1611, 1546 (C
O), 1611, 1546 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1469, 1428 (C–H aliph.), 1414, 1107 (Si–C6H5), 892, 859 (arom. 1,2,4-subst.), 743, 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.91 (d,J = 6.6 Hz,6H,26), 0.94 (d, J = 6.6 Hz,6H,27), 1.55 (m,2H,25), 1.97 (m,2H,17), 2.34 (m,2H,17′), 4.93 (dd,J = 11.5,4.3 Hz,2H,9), 7.60 (m,10H,13,14,15), 7.99 (d,J = 7.4 Hz,4H,6), 8.03 (s,2H,2), 8.14 (d,J = 7.4 Hz,4H,5). 13C NMR (acetone-d6) (δ) (ppm): 21.3 (26), 23.5 (27), 27.5 (25), 38.0 (17), 51.2 (9), 123.6 (5), 129.5 (14), 130.9 (15), 131.6 (2), 132.1 (3), 132.2 (4), 134.2 (12), 137.1 (13), 142.6 (6), 143.3 (1), 168.3, 168.4 (7,8), 171.2 (10). 29Si NMR (acetone-d6) (δ) (ppm): −13.54.
C arom.), 1469, 1428 (C–H aliph.), 1414, 1107 (Si–C6H5), 892, 859 (arom. 1,2,4-subst.), 743, 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.91 (d,J = 6.6 Hz,6H,26), 0.94 (d, J = 6.6 Hz,6H,27), 1.55 (m,2H,25), 1.97 (m,2H,17), 2.34 (m,2H,17′), 4.93 (dd,J = 11.5,4.3 Hz,2H,9), 7.60 (m,10H,13,14,15), 7.99 (d,J = 7.4 Hz,4H,6), 8.03 (s,2H,2), 8.14 (d,J = 7.4 Hz,4H,5). 13C NMR (acetone-d6) (δ) (ppm): 21.3 (26), 23.5 (27), 27.5 (25), 38.0 (17), 51.2 (9), 123.6 (5), 129.5 (14), 130.9 (15), 131.6 (2), 132.1 (3), 132.2 (4), 134.2 (12), 137.1 (13), 142.6 (6), 143.3 (1), 168.3, 168.4 (7,8), 171.2 (10). 29Si NMR (acetone-d6) (δ) (ppm): −13.54.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1608, 1543 (C
O), 1608, 1543 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1460, 1428 (C–H aliph.), 1414, 1106 (Si–C6H5), 849 (arom. 1,2,4-subst.), 742, 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.85 (t,6H,29), 1.08 (m,2H,28), 1.14 (d,J = 6.7 Hz,6H,23), 1.56 (m,2H,28′), 2.49 (m,2H,22), 4.64 (d,J = 8.1 Hz,2H,9), 7.54 (m,6H,14,15), 7.66 (d,4H,13), 8.00 (d,J = 7.4 Hz,2H,6), 8.03 (s,2H,2), 8.14 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): 11.3 (29), 17.2 (23), 26.5 (28), 35.5 (22), 57.5 (9), 123.7 (5), 129.5 (14), 131.0 (15), 131.6 (2), 132.0 (3), 132.1 (4), 134.1 (12), 137.1 (13), 142.7 (6), 143.3 (1), 168.3, 168.4 (7,8), 170.1 (10). 29Si NMR (acetone-d6) (δ) (ppm): −13.56.
C arom.), 1460, 1428 (C–H aliph.), 1414, 1106 (Si–C6H5), 849 (arom. 1,2,4-subst.), 742, 700 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.85 (t,6H,29), 1.08 (m,2H,28), 1.14 (d,J = 6.7 Hz,6H,23), 1.56 (m,2H,28′), 2.49 (m,2H,22), 4.64 (d,J = 8.1 Hz,2H,9), 7.54 (m,6H,14,15), 7.66 (d,4H,13), 8.00 (d,J = 7.4 Hz,2H,6), 8.03 (s,2H,2), 8.14 (d,J = 7.4 Hz,2H,5). 13C NMR (acetone-d6) (δ) (ppm): 11.3 (29), 17.2 (23), 26.5 (28), 35.5 (22), 57.5 (9), 123.7 (5), 129.5 (14), 131.0 (15), 131.6 (2), 132.0 (3), 132.1 (4), 134.1 (12), 137.1 (13), 142.7 (6), 143.3 (1), 168.3, 168.4 (7,8), 170.1 (10). 29Si NMR (acetone-d6) (δ) (ppm): −13.56.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1608, 1512 (C
O), 1608, 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 854 (arom. p-subst.), 1412, 1112 (Si–C6H5), 739, 702 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 7.60 (m,18H,13,14,15,19,20,21), 8.11 (m,6H,2,5,6), 9.46 (s,2H,OH). 13C NMR (acetone-d6) (δ) (ppm): 123.9 (5), 127.4 (31), 129.5 (14), 130.5 (33), 131.0 (32), 131.1 (15), 131.7 (2), 132.0 (3), 132.2 (4), 134.3 (12), 137.1 (13), 137.2 (30), 142.7 (6), 143.3 (1), 167.0, 167.3, 167.4 (7,8,10). 29Si NMR (acetone-d6) (δ) (ppm): −13.53.
C arom.), 854 (arom. p-subst.), 1412, 1112 (Si–C6H5), 739, 702 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 7.60 (m,18H,13,14,15,19,20,21), 8.11 (m,6H,2,5,6), 9.46 (s,2H,OH). 13C NMR (acetone-d6) (δ) (ppm): 123.9 (5), 127.4 (31), 129.5 (14), 130.5 (33), 131.0 (32), 131.1 (15), 131.7 (2), 132.0 (3), 132.2 (4), 134.3 (12), 137.1 (13), 137.2 (30), 142.7 (6), 143.3 (1), 167.0, 167.3, 167.4 (7,8,10). 29Si NMR (acetone-d6) (δ) (ppm): −13.53.The diamine bis(4-aminophenyl)diphenylsilane was obtained from 4-bromo-N,N-bis(trimethylsilyl)aniline and dichlorodiphenylsilane, according to a reported procedure.51
Poly(imide-amides) (PIAs)
PIAs were obtained according to the following general procedure (Scheme 1).52,53 A mixture of 5 mmol of the diamine, 5 mmol of the dicarboxylic imido-acids, 0.24 g of CaCl2, 0.3 mL of triphenylphosphite, 1.2 mL of pyridine and 1.8 mL of N-methyl-2-pyrrolidone (NMP) was heated at 120 °C for 3 hours. After this time the mixture was poured in 300 mL of methanol, and the solid was filtered, washed several times with methanol, dried under vacuum until constant weight, and characterized.Series I (R1 = R2 = Me)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1591, 1522 (C
O), 1591, 1522 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1426 (C–H aliph.), 1249 (Si–CH3), 1426, 1109 (Si–C6H5), 812 (arom. p-subst.), 733, 702 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.75 (s,6H,11), 4.47 (s,4H,9), 7.54 (m,18H,36,37,40,41,42), 7.93 (d,2H,6), 8.06 (d,2H,5), 8.11 (s,2H,2), 10.48 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.42 (11), 40.8 (9), 118.8 (36), 122.5 (5), 128.0 (41), 128.2 (2), 130.8 (38), 132.5 (3), 133.8 (4), 134.4 (42), 135.7, 135.8 (37,39), 136.5 (40), 139.9 (35), 140.2 (6), 145.6 (1), 164.9 (10), 167.4, 167.6 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −4.63 (43), −15.40 (44).
C arom.), 1426 (C–H aliph.), 1249 (Si–CH3), 1426, 1109 (Si–C6H5), 812 (arom. p-subst.), 733, 702 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.75 (s,6H,11), 4.47 (s,4H,9), 7.54 (m,18H,36,37,40,41,42), 7.93 (d,2H,6), 8.06 (d,2H,5), 8.11 (s,2H,2), 10.48 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.42 (11), 40.8 (9), 118.8 (36), 122.5 (5), 128.0 (41), 128.2 (2), 130.8 (38), 132.5 (3), 133.8 (4), 134.4 (42), 135.7, 135.8 (37,39), 136.5 (40), 139.9 (35), 140.2 (6), 145.6 (1), 164.9 (10), 167.4, 167.6 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −4.63 (43), −15.40 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1516 (C
O), 1590, 1516 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1458 (C–H aliph.), 1250 (Si–CH3), 1428, 1109 (Si–C6H5), 814 (arom. p-subst.), 741, 701 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.74 (s,6H,11), 1.58 (d,6H,16), 4.94 (q,2H,9), 7.5 (m,18H,36,37,40,41,42), 7.90 (d,2H,6), 8.05 (m,4H,2,5), 9.99 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.45 (11), 15.0 (16), 48.7 (9), 119.4 (36), 122.3 (5), 127.6 (2), 128.0 (41), 131.0 (38), 132.7 (3), 133.9 (4), 134.4 (42), 135.6 (37), 135.5 (39),136.2 (40), 140.0 (35), 140.1 (6), 145.3 (1), 167.3 (10), 167.5, 167.7 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −4.25 (43), −15.30 (44).
C arom.), 1458 (C–H aliph.), 1250 (Si–CH3), 1428, 1109 (Si–C6H5), 814 (arom. p-subst.), 741, 701 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.74 (s,6H,11), 1.58 (d,6H,16), 4.94 (q,2H,9), 7.5 (m,18H,36,37,40,41,42), 7.90 (d,2H,6), 8.05 (m,4H,2,5), 9.99 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.45 (11), 15.0 (16), 48.7 (9), 119.4 (36), 122.3 (5), 127.6 (2), 128.0 (41), 131.0 (38), 132.7 (3), 133.9 (4), 134.4 (42), 135.6 (37), 135.5 (39),136.2 (40), 140.0 (35), 140.1 (6), 145.3 (1), 167.3 (10), 167.5, 167.7 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −4.25 (43), −15.30 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1515 (C
O), 1590, 1515 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1428, 1318 (C–H aliph.), 832 (arom. p-subst.), 1249 (Si–CH3), 1428, 1108 (Si–C6H5), 739, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.71 (s,6H,11), 3.41 (d,2H,17), 3.65 (d,2H,17′), 5.26 (q,2H,9), 7.43 (m,28H,19,20,21,36,37,40,41,42), 7.85 (d,2H,6), 8.00 (m,4H,2,5), 10.16 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.1 (11), 34.5 (17), 55.2 (9), 120.1 (36), 122.8 (5), 126.9 (21), 128.1 (2), 128.4 (41), 128.6 (19), 129.2 (20), 130.8 (38), 132.5 (3), 134.3 (4), 134.9 (42), 136.1 (37), 136.1 (39), 136.7 (40), 137.8 (18), 140.4 (35), 140.5 (6), 145.9 (1), 167.2 (10), 167.7, 167.9 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −4.63 (43), −15.43 (44).
C arom.), 1428, 1318 (C–H aliph.), 832 (arom. p-subst.), 1249 (Si–CH3), 1428, 1108 (Si–C6H5), 739, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.71 (s,6H,11), 3.41 (d,2H,17), 3.65 (d,2H,17′), 5.26 (q,2H,9), 7.43 (m,28H,19,20,21,36,37,40,41,42), 7.85 (d,2H,6), 8.00 (m,4H,2,5), 10.16 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.1 (11), 34.5 (17), 55.2 (9), 120.1 (36), 122.8 (5), 126.9 (21), 128.1 (2), 128.4 (41), 128.6 (19), 129.2 (20), 130.8 (38), 132.5 (3), 134.3 (4), 134.9 (42), 136.1 (37), 136.1 (39), 136.7 (40), 137.8 (18), 140.4 (35), 140.5 (6), 145.9 (1), 167.2 (10), 167.7, 167.9 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −4.63 (43), −15.43 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1591, 1519 (C
O), 1591, 1519 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1469 (C–H aliph.), 1251 (Si–CH3), 1428, 1110 (Si–C6H5), 814 (arom. p-subst.), 739, 701 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.74 (s,6H,11), 0.85 (s,6H,23), 1.04 (s,6H,24), 2.82 (m,2H,22), 4.57 (m,2H,9), 7.51 (m,18H,36,37,40,41,42), 7.93 (s,2H,6) 8.09 (s,4H,2,5), 10.08 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.49 (11), 19.01 (23), 20.4 (24), 27.5 (22), 59.2 (9), 119.4 (36), 124.4 (5), 127.6 (2), 127.9 (41), 130.5 (38), 132.2 (3), 133.8 (4), 134.4 (42), 135.6 (37), 135.6 (39), 136.2 (40), 138.5 (35), 140.1 (6), 145.4 (1), 166.7 (10), 167.6, 167.8 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −4.57 (43), −15.33 (44).
C arom.), 1469 (C–H aliph.), 1251 (Si–CH3), 1428, 1110 (Si–C6H5), 814 (arom. p-subst.), 739, 701 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.74 (s,6H,11), 0.85 (s,6H,23), 1.04 (s,6H,24), 2.82 (m,2H,22), 4.57 (m,2H,9), 7.51 (m,18H,36,37,40,41,42), 7.93 (s,2H,6) 8.09 (s,4H,2,5), 10.08 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.49 (11), 19.01 (23), 20.4 (24), 27.5 (22), 59.2 (9), 119.4 (36), 124.4 (5), 127.6 (2), 127.9 (41), 130.5 (38), 132.2 (3), 133.8 (4), 134.4 (42), 135.6 (37), 135.6 (39), 136.2 (40), 138.5 (35), 140.1 (6), 145.4 (1), 166.7 (10), 167.6, 167.8 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −4.57 (43), −15.33 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1516 (C
O), 1590, 1516 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1469 (C–H aliph.), 1250 (Si–CH3), 1428, 1109 (Si–C6H5), 833 (arom. p-subst.), 740, 701 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.76 (s,6H,11), 0.89 (s,6H,26), 0.92 (s,6H,27), 1.44 (m,2H,25), 1.98 (m,2H,17), 2.24 (m,2H,17′), 4.95 (m,2H,9), 7.52 (m,18H,36,37,40,41,42), 7.95 (s,2H,6), 8.11 (s,4H,2,5), 10.06 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.49 (11), 20.7 (26), 23.1 (27), 24.6 (25), 36.8 (17), 52.1 (9), 119.6 (36), 122.4 (5), 127.6 (2), 128.0 (41), 130.7 (38), 132.3 (3), 133.8 (4), 134.4 (42), 135.6, 135.7 (37,39), 136.2 (40), 140.0 (35), 140.1 (6), 145.5 (1), 167.5 (10), 167.6, 167.8 (7,8). 29Si NMR (acetone-d6) (δ) (ppm): −4.59 (43), −15.30 (44).
C arom.), 1469 (C–H aliph.), 1250 (Si–CH3), 1428, 1109 (Si–C6H5), 833 (arom. p-subst.), 740, 701 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.76 (s,6H,11), 0.89 (s,6H,26), 0.92 (s,6H,27), 1.44 (m,2H,25), 1.98 (m,2H,17), 2.24 (m,2H,17′), 4.95 (m,2H,9), 7.52 (m,18H,36,37,40,41,42), 7.95 (s,2H,6), 8.11 (s,4H,2,5), 10.06 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.49 (11), 20.7 (26), 23.1 (27), 24.6 (25), 36.8 (17), 52.1 (9), 119.6 (36), 122.4 (5), 127.6 (2), 128.0 (41), 130.7 (38), 132.3 (3), 133.8 (4), 134.4 (42), 135.6, 135.7 (37,39), 136.2 (40), 140.0 (35), 140.1 (6), 145.5 (1), 167.5 (10), 167.6, 167.8 (7,8). 29Si NMR (acetone-d6) (δ) (ppm): −4.59 (43), −15.30 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1517 (C
O), 1590, 1517 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1461, 1428, 1373 (C–H aliph.), 1251 (Si–CH3), 1428, 1108 (Si–C6H5), 833 (arom. p-subst.), 738, 699 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.75 (s,6H,11), 0.83 (s,6H,29), 1.03 (s,6H,23), 1.09 (m,2H,22), 1.49 (m,2H,28), 2.71 (m,2H,28), 4.67 (d,2H,9), 7.53 (m,18H,36,37,40,41,42), 7.93 (s,2H,6), 8.09 (s,4H,2,5), 10.91 (s,2H,34). 13C NMR (acetone-d6) (δ) (ppm): −3.43 (11), 10.7 (29), 16.3 (23), 25.2 (28), 33.2 (23), 58.9 (9), 119.4 (35), 122.5 (5), 127.7 (2), 128.0 (41), 130.5 (38), 132.2 (3), 134.0 (4), 134.5 (42), 135.7 (37), 135.7 (39), 136.3 (40), 140.0 (35), 140.2 (6), 145.5 (1), 167.0 (10), 167.7, 167.9 (7,8). 29Si NMR (acetone-d6) (δ) (ppm): −4.63 (43), −15.43 (44).
C arom.), 1461, 1428, 1373 (C–H aliph.), 1251 (Si–CH3), 1428, 1108 (Si–C6H5), 833 (arom. p-subst.), 738, 699 (arom. mono-subst.). 1H NMR (acetone-d6) (δ) (ppm): 0.75 (s,6H,11), 0.83 (s,6H,29), 1.03 (s,6H,23), 1.09 (m,2H,22), 1.49 (m,2H,28), 2.71 (m,2H,28), 4.67 (d,2H,9), 7.53 (m,18H,36,37,40,41,42), 7.93 (s,2H,6), 8.09 (s,4H,2,5), 10.91 (s,2H,34). 13C NMR (acetone-d6) (δ) (ppm): −3.43 (11), 10.7 (29), 16.3 (23), 25.2 (28), 33.2 (23), 58.9 (9), 119.4 (35), 122.5 (5), 127.7 (2), 128.0 (41), 130.5 (38), 132.2 (3), 134.0 (4), 134.5 (42), 135.7 (37), 135.7 (39), 136.3 (40), 140.0 (35), 140.2 (6), 145.5 (1), 167.0 (10), 167.7, 167.9 (7,8). 29Si NMR (acetone-d6) (δ) (ppm): −4.63 (43), −15.43 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589, 1507 (C
O), 1589, 1507 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1440 (C–H aliph.), 1282 (Si–CH3), 1427, 1111 (Si–C6H5), 833 (arom. p-subst.), 738, 701 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.80 (s,6H,11), 7.80 (m,32H,2,5,6,31,32,36,37,40,41,42), 10.52 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.4 (11), 119.7 (36), 120.2 (33), 122.7 (5), 126.8 (31), 128.0 (41), 128.2 (32), 128.5 (2), 130.7 (38), 132.4 (3), 134.0 (4), 134.2 (30), 134.4 (42), 135.7 (37), 135.7 (39), 136.3 (40), 140.2 (35), 140.5 (6), 145.6 (1), 165.1 (10), 166.6, 166.8 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −4.63 (43), −15.43 (44).
C arom.), 1440 (C–H aliph.), 1282 (Si–CH3), 1427, 1111 (Si–C6H5), 833 (arom. p-subst.), 738, 701 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.80 (s,6H,11), 7.80 (m,32H,2,5,6,31,32,36,37,40,41,42), 10.52 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.4 (11), 119.7 (36), 120.2 (33), 122.7 (5), 126.8 (31), 128.0 (41), 128.2 (32), 128.5 (2), 130.7 (38), 132.4 (3), 134.0 (4), 134.2 (30), 134.4 (42), 135.7 (37), 135.7 (39), 136.3 (40), 140.2 (35), 140.5 (6), 145.6 (1), 165.1 (10), 166.6, 166.8 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −4.63 (43), −15.43 (44).Series II (R1 = Me, R2 = C6H5)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1517 (C
O), 1590, 1517 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1426, 1390 (C–H aliph.), 1243 (Si–CH3), 1426, 1109 (Si–C6H5), 822 (arom. p-subst.), 734, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 1.08 (s,3H,11), 4.51 (s,4H,9), 7.57 (m,23H,13,14,15,36,37,40,41,42), 8.02 (m,6H,2,5,6), 10.52 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −4.4 (11), 40.8 (9), 118.8 (37), 122.7 (5), 127.7 (14), 128.0 (41), 128.4 (2), 128.8 (15), 130.9 (38), 132.8 (3), 132.9 (12), 133.8 (4), 134.4 (42), 135.0 (13), 135.7 (37), 135.7 (39), 136.5 (40), 139.9 (35), 141.1 (6), 143.3 (1), 164.9 (10), 167.3, 167.5 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.91 (43), −15.39 (44).
C arom.), 1426, 1390 (C–H aliph.), 1243 (Si–CH3), 1426, 1109 (Si–C6H5), 822 (arom. p-subst.), 734, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 1.08 (s,3H,11), 4.51 (s,4H,9), 7.57 (m,23H,13,14,15,36,37,40,41,42), 8.02 (m,6H,2,5,6), 10.52 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −4.4 (11), 40.8 (9), 118.8 (37), 122.7 (5), 127.7 (14), 128.0 (41), 128.4 (2), 128.8 (15), 130.9 (38), 132.8 (3), 132.9 (12), 133.8 (4), 134.4 (42), 135.0 (13), 135.7 (37), 135.7 (39), 136.5 (40), 139.9 (35), 141.1 (6), 143.3 (1), 164.9 (10), 167.3, 167.5 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.91 (43), −15.39 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589, 1512 (C
O), 1589, 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1443, 1427 (C–H aliph.), 1428, 1109 (Si–C6H5), 822 (arom. p-subst.), 740, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 1.06 (s,3H,11), 1.61 (d,6H,16), 4.97 (q,2H,9), 7.59 (m,23H,13–15,36,37,40,41,42), 7.98 (m,6H,2,5,6), 10.02 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −4.4 (11), 15.0 (16), 48.9 (9), 119.5 (36), 122.6 (5), 127.6 (14), 128.0 (41), 128.4 (5), 128.6 (15), 131.2 (38), 132.9 (3), 133.1 (12), 134.0 (4), 134.5 (42), 134.9 (13), 135.7 (37), 135.7 (39), 136.3 (40), 140.1 (35), 140.9 (6), 143.0 (1), 167.2 (10), 167.4, 167.7 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.21 (43), −15.19 (44).
C arom.), 1443, 1427 (C–H aliph.), 1428, 1109 (Si–C6H5), 822 (arom. p-subst.), 740, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 1.06 (s,3H,11), 1.61 (d,6H,16), 4.97 (q,2H,9), 7.59 (m,23H,13–15,36,37,40,41,42), 7.98 (m,6H,2,5,6), 10.02 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −4.4 (11), 15.0 (16), 48.9 (9), 119.5 (36), 122.6 (5), 127.6 (14), 128.0 (41), 128.4 (5), 128.6 (15), 131.2 (38), 132.9 (3), 133.1 (12), 134.0 (4), 134.5 (42), 134.9 (13), 135.7 (37), 135.7 (39), 136.3 (40), 140.1 (35), 140.9 (6), 143.0 (1), 167.2 (10), 167.4, 167.7 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.21 (43), −15.19 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589, 1511 (C
O), 1589, 1511 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1454, 1428 (C–H aliph.), 1241 (Si–CH3), 1428, 1108 (Si–C6H5), 821 (arom. p-subst.), 739, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 1.02 (s,3H,11), 3.41 (d,2H,17), 3.64 (d,2H,17′), 5.26 (q,2H,9), 7.19 (m,5H,13,14,15), 7.46 (m,24H,19,10,21,36,40,41,42), 7.69 (m,4H,37), 7.90 (m,6H,2,5,6), 10.16 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −4.07 (CH3,11), 34.6 (CH2,17), 55.4 (9), 120.2 (36), 123.0 (5), 126.9 (21), 128.5 (14), 128.5 (41), 128.6 (5), 128.6 (20), 128.8 (15), 129.2 (19), 131.1 (38), 132.9 (3), 133.2 (12), 134.4 (4), 135.2 (42), 135.4 (13), 136.2 (37), 136.2 (39), 136.8 (40), 137.8 (18), 140.3 (35), 141.6 (6), 143.7 (1), 167.2 (10), 167.7, 167.9 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.50 (43), −15.25 (44).
C arom.), 1454, 1428 (C–H aliph.), 1241 (Si–CH3), 1428, 1108 (Si–C6H5), 821 (arom. p-subst.), 739, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 1.02 (s,3H,11), 3.41 (d,2H,17), 3.64 (d,2H,17′), 5.26 (q,2H,9), 7.19 (m,5H,13,14,15), 7.46 (m,24H,19,10,21,36,40,41,42), 7.69 (m,4H,37), 7.90 (m,6H,2,5,6), 10.16 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −4.07 (CH3,11), 34.6 (CH2,17), 55.4 (9), 120.2 (36), 123.0 (5), 126.9 (21), 128.5 (14), 128.5 (41), 128.6 (5), 128.6 (20), 128.8 (15), 129.2 (19), 131.1 (38), 132.9 (3), 133.2 (12), 134.4 (4), 135.2 (42), 135.4 (13), 136.2 (37), 136.2 (39), 136.8 (40), 137.8 (18), 140.3 (35), 141.6 (6), 143.7 (1), 167.2 (10), 167.7, 167.9 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.50 (43), −15.25 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1516 (C
O), 1590, 1516 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1443, 1372 (C–H aliph.), 1284 (Si–CH3), 1428, 1109 (Si–C6H5), 823 (arom. p-subst.), 737, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.88 (s,3H,11), 1.06 (d,12H,23,24), 2.85 (m,2H,22), 4.60 (s,2H,9), 7.53 (m,23H,13,14,15,36,40,41,42), 8.00 (m,6H,2,5,6), 10.12 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −4.39 (11), 19.2 (23), 20.4 (24), 27.5 (22), 59.4 (9), 119.4 (36), 122.7 (5), 127.7 (14), 128.0 (41), 128.4 (2), 128.8 (15), 130.7 (38), 132.6 (3), 132.9 (12), 134.0 (4), 134.5 (42), 134.9 (13), 135.7 (37), 135.7 (39), 136.3 (40), 140.1 (35), 141.1 (6), 143.2 (1), 166.7 (10), 167.6, 167.8 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.91 (43), −15.39 (44).
C arom.), 1443, 1372 (C–H aliph.), 1284 (Si–CH3), 1428, 1109 (Si–C6H5), 823 (arom. p-subst.), 737, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.88 (s,3H,11), 1.06 (d,12H,23,24), 2.85 (m,2H,22), 4.60 (s,2H,9), 7.53 (m,23H,13,14,15,36,40,41,42), 8.00 (m,6H,2,5,6), 10.12 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −4.39 (11), 19.2 (23), 20.4 (24), 27.5 (22), 59.4 (9), 119.4 (36), 122.7 (5), 127.7 (14), 128.0 (41), 128.4 (2), 128.8 (15), 130.7 (38), 132.6 (3), 132.9 (12), 134.0 (4), 134.5 (42), 134.9 (13), 135.7 (37), 135.7 (39), 136.3 (40), 140.1 (35), 141.1 (6), 143.2 (1), 166.7 (10), 167.6, 167.8 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.91 (43), −15.39 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1588, 1511 (C
O), 1588, 1511 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1455, 1428 (C–H aliph.), 1240 (Si–CH3), 1428, 1108 (Si–C6H5), 822 (arom. p-subst), 739, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.89 (s,6H,26), 0.93 (s,6H,27), 1.08 (s,3H,11), 1.47 (m,2H,25), 2.01 (t,2H,17), 2.26 (t,2H,17′), 4.96 (d,2H,9), 7.55 (m,23H,13,14,15,36,40,41,42), 8.02 (m,6H,2,5,6), 10.09 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −4.1 (11), 21.1 (26), 23.5 (27), 25.0 (25), 37.2 (17), 52.5 (9), 120.0 (36), 123.0 (5), 128.2 (14), 128.3 (41), 128.7 (2), 128.8 (15), 131.2 (38), 133.1 (3), 133.2 (12), 134.2 (4), 135.0 (42), 135.3 (13), 136.0 (37), 136.0 (39), 136.6 (40), 140.4 (35), 141.5 (6), 143.6 (1), 167.8 (10), 167.9, 168.1 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.90 (43), −15.39 (44).
C arom.), 1455, 1428 (C–H aliph.), 1240 (Si–CH3), 1428, 1108 (Si–C6H5), 822 (arom. p-subst), 739, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.89 (s,6H,26), 0.93 (s,6H,27), 1.08 (s,3H,11), 1.47 (m,2H,25), 2.01 (t,2H,17), 2.26 (t,2H,17′), 4.96 (d,2H,9), 7.55 (m,23H,13,14,15,36,40,41,42), 8.02 (m,6H,2,5,6), 10.09 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −4.1 (11), 21.1 (26), 23.5 (27), 25.0 (25), 37.2 (17), 52.5 (9), 120.0 (36), 123.0 (5), 128.2 (14), 128.3 (41), 128.7 (2), 128.8 (15), 131.2 (38), 133.1 (3), 133.2 (12), 134.2 (4), 135.0 (42), 135.3 (13), 136.0 (37), 136.0 (39), 136.6 (40), 140.4 (35), 141.5 (6), 143.6 (1), 167.8 (10), 167.9, 168.1 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.90 (43), −15.39 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1512 (C
O), 1590, 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1462, 1443, 1360 (C–H aliph.), 1280 (Si–CH3), 1428, 1109 (Si–C6H5), 822 (arom. p-subst.), 737, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.85 (s,3H,11), 0.92 (m,2H,28), 1.04 (m,12H,23,29), 1.51 (m,2H,28), 2.73 (m,2H,22), 4.69 (d,2H,9), 7.55 (m,23H,13,14,15,36,40,41,42), 8.00 (m,6H,2,5,6), 10.22 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −4.90 (11), 10.7 (29), 16.2 (23), 25.2 (28), 33.2 (22), 59.0 (9), 119.3 (36), 122.7 (5), 127.7 (14), 128.0 (41), 128.4 (2), 128.8 (15), 130.6 (38), 132.5 (3), 132.9 (12), 134.0 (4), 134.5 (42), 135.0 (13), 135.7 (37), 135.7 (39), 136.4 (40), 140.0 (35), 141.2 (6), 143.3 (1), 167.0 (10), 167.7, 167.9 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.91 (43), −15.39 (44).
C arom.), 1462, 1443, 1360 (C–H aliph.), 1280 (Si–CH3), 1428, 1109 (Si–C6H5), 822 (arom. p-subst.), 737, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.85 (s,3H,11), 0.92 (m,2H,28), 1.04 (m,12H,23,29), 1.51 (m,2H,28), 2.73 (m,2H,22), 4.69 (d,2H,9), 7.55 (m,23H,13,14,15,36,40,41,42), 8.00 (m,6H,2,5,6), 10.22 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −4.90 (11), 10.7 (29), 16.2 (23), 25.2 (28), 33.2 (22), 59.0 (9), 119.3 (36), 122.7 (5), 127.7 (14), 128.0 (41), 128.4 (2), 128.8 (15), 130.6 (38), 132.5 (3), 132.9 (12), 134.0 (4), 134.5 (42), 135.0 (13), 135.7 (37), 135.7 (39), 136.4 (40), 140.0 (35), 141.2 (6), 143.3 (1), 167.0 (10), 167.7, 167.9 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.91 (43), −15.39 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1588, 1560 (C
O), 1588, 1560 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1439 (C–H aliph), 1281 (Si–CH3), 1428, 1111 (Si–C6H5), 824 (arom. p-subst.), 735, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 1.11 (s,3H,11), 7.77 (m,37H,2,5,6,13,14,15,31,32,36,40,41,42), 10.52 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.9 (11), 120.2 (36), 123.4 (5), 127.4 (31), 128.1 (14), 128.5 (32), 128.7 (41), 128.9 (2), 129.0 (15), 131.4 (13), 133.3 (3), 133.4 (12), 134.4 (4), 134.7 (33), 134.9 (42), 135.1 (30), 135.4 (13), 136.2 (37),136.2 (39), 136.8 (40), 141.0 (35), 141.7 (6), 143.8 (1), 165.6 (10), 167.0, 167.2 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.91 (43), −15.39 (44).
C arom.), 1439 (C–H aliph), 1281 (Si–CH3), 1428, 1111 (Si–C6H5), 824 (arom. p-subst.), 735, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 1.11 (s,3H,11), 7.77 (m,37H,2,5,6,13,14,15,31,32,36,40,41,42), 10.52 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): −3.9 (11), 120.2 (36), 123.4 (5), 127.4 (31), 128.1 (14), 128.5 (32), 128.7 (41), 128.9 (2), 129.0 (15), 131.4 (13), 133.3 (3), 133.4 (12), 134.4 (4), 134.7 (33), 134.9 (42), 135.1 (30), 135.4 (13), 136.2 (37),136.2 (39), 136.8 (40), 141.0 (35), 141.7 (6), 143.8 (1), 165.6 (10), 167.0, 167.2 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −8.91 (43), −15.39 (44).Series III (R1 = R2 = C6H5)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1517 (C
O), 1590, 1517 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1426 (C–H aliph.), 1427, 1109 (Si–C6H5), 822 (arom. p-subst.), 729, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 4.48 (s,4H,9), 7.53 (m,28H,13,14,15,36,40,41,42), 7.91 (s,2H,6), 8.05 (s,4H,2,5), 10.49 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 41.1 (9), 119.0 (36), 123.3 (5), 128.3 (41), 128.9 (14), 129.6 (2), 129.9 (15), 131.0 (4), 131.3 (3), 133.4 (38), 134.1 (12), 134.7 (42), 135.9 (37), 135.9 (39), 136.1 (13), 136.8 (40), 140.1 (35), 141.2 (6), 142.4 (1), 165.2 (10), 167.5, 167.7 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −14.56 (43), −16.05 (44).
C arom.), 1426 (C–H aliph.), 1427, 1109 (Si–C6H5), 822 (arom. p-subst.), 729, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 4.48 (s,4H,9), 7.53 (m,28H,13,14,15,36,40,41,42), 7.91 (s,2H,6), 8.05 (s,4H,2,5), 10.49 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 41.1 (9), 119.0 (36), 123.3 (5), 128.3 (41), 128.9 (14), 129.6 (2), 129.9 (15), 131.0 (4), 131.3 (3), 133.4 (38), 134.1 (12), 134.7 (42), 135.9 (37), 135.9 (39), 136.1 (13), 136.8 (40), 140.1 (35), 141.2 (6), 142.4 (1), 165.2 (10), 167.5, 167.7 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −14.56 (43), −16.05 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1511 (C
O), 1590, 1511 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1444, 1375 (C–H aliph.), 1428, 1109 (Si–C6H5), 822 (arom. p-subst.), 742, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 1.60 (d,6H,16), 4.97 (q,2H,9), 7.53 (m,28H,13,14,15,36,40,41,42), 7.88 (s,2H,6), 8.04 (s,4H,2,5), 10,02 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 15.4 (16), 42.3 (9), 120.0 (36), 123.3 (5), 128.4 (41), 129.1 (14), 129.6 (2), 130.2 (15), 131.2 (4), 131.7 (3), 133.8 (38), 134.3 (12), 134.8 (42), 136.1 (37),136.1 (39), 136.2 (13), 136.7 (40), 140.5 (35), 141.1 (6), 142.3 (1), 167.5 (10), 167.7, 168.0 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −14.24 (43), −15.66 (44).
C arom.), 1444, 1375 (C–H aliph.), 1428, 1109 (Si–C6H5), 822 (arom. p-subst.), 742, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 1.60 (d,6H,16), 4.97 (q,2H,9), 7.53 (m,28H,13,14,15,36,40,41,42), 7.88 (s,2H,6), 8.04 (s,4H,2,5), 10,02 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 15.4 (16), 42.3 (9), 120.0 (36), 123.3 (5), 128.4 (41), 129.1 (14), 129.6 (2), 130.2 (15), 131.2 (4), 131.7 (3), 133.8 (38), 134.3 (12), 134.8 (42), 136.1 (37),136.1 (39), 136.2 (13), 136.7 (40), 140.5 (35), 141.1 (6), 142.3 (1), 167.5 (10), 167.7, 168.0 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −14.24 (43), −15.66 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589, 1510 (C
O), 1589, 1510 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1455 (C–H aliph.), 1428, 1108 (Si–C6H5), 820 (arom. p-subst.), 740, 699 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 3.43 (d,2H,17), 3.63 (d,2H,17′), 5.25 (d,2H,9), 7.43 (m,36H,13,14,15,36,40,41,42), 7.68 (d,4H,19), 7.78 (d,2H,6), 7.97 (d,4H,2,5), 10.16 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 34.1 (17), 55.0 (9), 119.7 (36), 122.9 (5), 126.5 (21), 128.0 (41), 128.2 (20), 128.6 (14), 128.8 (13), 129.2 (2), 129.7 (15), 130.6 (4), 130.7 (3), 132.7 (38), 133.9 (12), 134.5 (42), 135.7 (37), 135.7 (39), 135.8 (13), 136.3 (18), 137.3 (40), 140.0 (35), 140.9 (6), 142.0 (1), 166.7 (10), 167.1, 167.3 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −14.09 (43), −15.33 (44).
C arom.), 1455 (C–H aliph.), 1428, 1108 (Si–C6H5), 820 (arom. p-subst.), 740, 699 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 3.43 (d,2H,17), 3.63 (d,2H,17′), 5.25 (d,2H,9), 7.43 (m,36H,13,14,15,36,40,41,42), 7.68 (d,4H,19), 7.78 (d,2H,6), 7.97 (d,4H,2,5), 10.16 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 34.1 (17), 55.0 (9), 119.7 (36), 122.9 (5), 126.5 (21), 128.0 (41), 128.2 (20), 128.6 (14), 128.8 (13), 129.2 (2), 129.7 (15), 130.6 (4), 130.7 (3), 132.7 (38), 133.9 (12), 134.5 (42), 135.7 (37), 135.7 (39), 135.8 (13), 136.3 (18), 137.3 (40), 140.0 (35), 140.9 (6), 142.0 (1), 166.7 (10), 167.1, 167.3 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −14.09 (43), −15.33 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589, 1506 (C
O), 1589, 1506 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1443, 1370 (C–H aliph.), 1428, 1109 (Si–C6H5), 821 (arom. p-subst.), 740, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.88 (d,6H,23), 1.05 (d,6H,24), 2.84 (m,2H,22), 4.59 (d,2H,9), 7.52 (m,28H,13,14,15,36,40,41,42), 7.90 (s,2H,6), 8.05 (d,4H,2,5), 10.11 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 19.2 (23), 20.4 (24), 27.5 (22), 59.5 (9), 119.4 (36), 122.9 (5), 127.6 (41), 128.0 (14), 128.1 (2), 128.6 (15), 130.9 (4), 131.1 (3), 132.8 (38), 133.4 (12), 134.4 (42), 135.7 (37), 135.7 (39), 135.9 (13), 136.3 (40), 140.0 (35), 140.9 (6), 142.1 (1), 166.6 (10), 167.5, 167.7 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −13.84 (43), −15.33 (44).
C arom.), 1443, 1370 (C–H aliph.), 1428, 1109 (Si–C6H5), 821 (arom. p-subst.), 740, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.88 (d,6H,23), 1.05 (d,6H,24), 2.84 (m,2H,22), 4.59 (d,2H,9), 7.52 (m,28H,13,14,15,36,40,41,42), 7.90 (s,2H,6), 8.05 (d,4H,2,5), 10.11 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 19.2 (23), 20.4 (24), 27.5 (22), 59.5 (9), 119.4 (36), 122.9 (5), 127.6 (41), 128.0 (14), 128.1 (2), 128.6 (15), 130.9 (4), 131.1 (3), 132.8 (38), 133.4 (12), 134.4 (42), 135.7 (37), 135.7 (39), 135.9 (13), 136.3 (40), 140.0 (35), 140.9 (6), 142.1 (1), 166.6 (10), 167.5, 167.7 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −13.84 (43), −15.33 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1511 (C
O), 1590, 1511 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1428, 1368 (C–H aliph.), 1428, 1109 (Si–C6H5), 822 (arom. p-subst.), 740, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.90 (d,12H,26,27), 1.48 (m,2H,25), 1.94 (m,2H,17), 2.32 (m,2H,17′), 4.95 (d,2H,9), 7.50 (m,28H,1,14,15,36,40,41,42), 7.90 (s,2H,6), 8.06 (d,4H,2,5), 10.08 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 18.5 (26), 20.8 (27), 23.1 (25), 36.9 (17), 52.2 (9), 119.6 (36), 123.0 (5), 127.7 (41), 128.0 (14), 128.6 (2), 129.5 (15), 130.8 (4), 131.0 (3), 133.0 (38), 133.9 (12), 134.5 (42), 135.7 (37), 135.7 (39), 135.9 (13), 136.3 (40), 140.0 (35), 142.1 (6), 143.4 (1), 167.5 (10), 167.7, 167.8 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −13.91 (43), −15.29 (44).
C arom.), 1428, 1368 (C–H aliph.), 1428, 1109 (Si–C6H5), 822 (arom. p-subst.), 740, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.90 (d,12H,26,27), 1.48 (m,2H,25), 1.94 (m,2H,17), 2.32 (m,2H,17′), 4.95 (d,2H,9), 7.50 (m,28H,1,14,15,36,40,41,42), 7.90 (s,2H,6), 8.06 (d,4H,2,5), 10.08 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 18.5 (26), 20.8 (27), 23.1 (25), 36.9 (17), 52.2 (9), 119.6 (36), 123.0 (5), 127.7 (41), 128.0 (14), 128.6 (2), 129.5 (15), 130.8 (4), 131.0 (3), 133.0 (38), 133.9 (12), 134.5 (42), 135.7 (37), 135.7 (39), 135.9 (13), 136.3 (40), 140.0 (35), 142.1 (6), 143.4 (1), 167.5 (10), 167.7, 167.8 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −13.91 (43), −15.29 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1510 (C
O), 1590, 1510 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1462, 1442, 1361 (C–H aliph.), 1428, 1109 (Si–C6H5), 821 (arom. p-subst.), 740, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.85 (s,6H,29), 0.86 (m,2H,28), 1.03 (s,6H,23), 1.52 (m,2H,28), 2.73 (m,2H,22), 4.69 (d,2H,9), 7.54 (m,28H,13,14,15,36,40,41,42), 7.90 (s,2H,6), 8.05 (d,4H,2,5), 10.20 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 10.7 (29), 16.2 (23), 18.5 (28), 25.2 (22), 59.1 (9), 119.3 (35), 122.9 (5), 127.7 (41), 128.1 (14), 128.6 (2), 129.5 (15), 130.7 (4), 130.8 (3), 132.8 (38), 133.9 (12), 134.5 (42), 135.7 (37), 135.7 (39), 135.9 (13), 136.4 (40), 140.0 (35), 140.9 (6), 142.1 (1), 166.9 (10), 167.6, 167.8 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −13.92 (43), −15.31 (44).
C arom.), 1462, 1442, 1361 (C–H aliph.), 1428, 1109 (Si–C6H5), 821 (arom. p-subst.), 740, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.85 (s,6H,29), 0.86 (m,2H,28), 1.03 (s,6H,23), 1.52 (m,2H,28), 2.73 (m,2H,22), 4.69 (d,2H,9), 7.54 (m,28H,13,14,15,36,40,41,42), 7.90 (s,2H,6), 8.05 (d,4H,2,5), 10.20 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 10.7 (29), 16.2 (23), 18.5 (28), 25.2 (22), 59.1 (9), 119.3 (35), 122.9 (5), 127.7 (41), 128.1 (14), 128.6 (2), 129.5 (15), 130.7 (4), 130.8 (3), 132.8 (38), 133.9 (12), 134.5 (42), 135.7 (37), 135.7 (39), 135.9 (13), 136.4 (40), 140.0 (35), 140.9 (6), 142.1 (1), 166.9 (10), 167.6, 167.8 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −13.92 (43), −15.31 (44).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1428, 1110 (Si–C6H5), 821 (arom. p-subst.), 739, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 7.80 (m,42H,2,5,6,13,14,15,31,32,36,37,40,41,42), 10.52 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 120.0 (36), 123.2 (5), 127.0 (31), 127.7 (2), 128.2 (14), 128.3 (15), 128.7 (41), 129.5 (33), 130.8 (32), 131.1, (4) 131.8 (3), 133.2 (38), 133.4 (12), 134.5 (42), 135.8 (13), 135.9 (37), 135.9 (39), 136.3 (40), 136.4 (30), 139.4 (35), 141.1 (6), 142.1 (1), 165.2 (10), 166.6, 166.7 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −13.90 (43), −15.03 (44).
O), 1428, 1110 (Si–C6H5), 821 (arom. p-subst.), 739, 700 (arom. mono-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 7.80 (m,42H,2,5,6,13,14,15,31,32,36,37,40,41,42), 10.52 (s,2H,34). 13C NMR (DMSO-d6) (δ) (ppm): 120.0 (36), 123.2 (5), 127.0 (31), 127.7 (2), 128.2 (14), 128.3 (15), 128.7 (41), 129.5 (33), 130.8 (32), 131.1, (4) 131.8 (3), 133.2 (38), 133.4 (12), 134.5 (42), 135.8 (13), 135.9 (37), 135.9 (39), 136.3 (40), 136.4 (30), 139.4 (35), 141.1 (6), 142.1 (1), 165.2 (10), 166.6, 166.7 (7,8). 29Si NMR (DMSO-d6) (δ) (ppm): −13.90 (43), −15.03 (44).Results and discussion
Tetramethyl derivatives (TM-(I–III)), tetracarboxylic imido-acids (TA-(I–III)) and dianhydrides (DA-(I–III)) were obtained according to described procedures and characterized by spectroscopic methods.43–47The monomeric dicarboxylic imido-acids (I-III-(a–g)) were obtained from the dianhydrides (DA-(I–III)) and the amino acids glycine, L-alanine, L-phenylalanine, L-valine, L-leucine, L-isoleucine, and p-aminobenzoic acid, in acetic acid according to describe procedures41,42,48,49 and characterized by IR, 1H, 13C and 29Si NMR, and when corresponds by optical activity. The results are summarized in the Experimental Part were in agreement with the proposed structures (Scheme 1).
In all the dicarboxylic imido-acids it was possible to see the IR band corresponding to the OH group between 3482 and 3466 cm−1 and a new band corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the acid.
O group of the acid.
In the NMR of both, 1H and 13C, it was possible to see the magnetic no-equivalence of the aliphatic groups of the amino acid residue. In fact, for the dicarboxylic imido-acids I-d, II-d and III-d derived from L-valine as amino acid, the two methyl groups were non-equivalent in 1H and 13C, due to the effect of the chiral carbon. The same effect was observed for the dicarboxylic imido-acids I-e, II-e and III-e, derived from L-leucine. In these dicarboxylic imido-acids the effect of the chiral carbon affects to the CH3 groups and to the CH2, in which the H atoms were non-equivalent in 1H NMR. Fig. 1 shows the NMR spectra of 1H, 13C and 29Si for the imido-diacid Id as example.
 | ||
| Fig. 1 Spectra for imido-diacid Id derived from L-valine (acetone-d6, 400 MHz). (a) NMR 1H, (b) NMR 13C and (c) NMR 29Si. | ||
In 13C NMR the effect was observed only for the CH3 groups. For the dicarboxylic imido-acids I-f, II-f and III-f derived from L-isoleucine, the non-equivalence was observed for the protons of the CH2 group in 1H NMR. This effect was described for us in other work.39
Respect to the 29Si NMR, it was possible to see the corresponding signal to the Si atom at high field. If the signal corresponding to the Si atom bonded to four aromatic rings (dicarboxylic imido-acids III-(a–g)) is considered like a reference about −13.5 ppm, the signal corresponding to the other dicarboxylic imido-acids II-(a–g) with one methyl group and three phenyl rings bonded to the Si atom, is shifted to lower field at about −9.0 ppm. Those corresponding to the dicarboxylic imido-acids I-(a–g), in which the Si atom is bonded to two methyl and two phenyl groups, the signal is shifted to about −4.5 ppm. In general the replacement of and aliphatic by an aromatic group, shifts the Si signal to higher field, due to the high electronic density of the Si atom with aromatic substituents.53–57
The poly(imide-amides) (PIAs) were obtained from the monomeric imido-diacids and the diamine bis(4-aminophenyl)diphenylsilane by direct polycondensation according to the Yamazaki method,52,53 in which the dicarboxylic imido-acid and the diamine were mixed with triphenylphosphite, CaCl2, pyridine and NMP (Scheme 1). The mixture was heated at 120 °C for three hours and then poured into methanol. The PIAs were filtered, washed with methanol, dried under vacuum until constant weight, and characterized.
In the IR spectra it was possible to see the disappearance of the band corresponding to the OH groups of the dicarboxylic imido-acids and a new band at about 3380 cm−1 corresponding to the NH group of the amides. The rest of the spectra were very similar to those obtained for the dicarboxylic imido-acids.
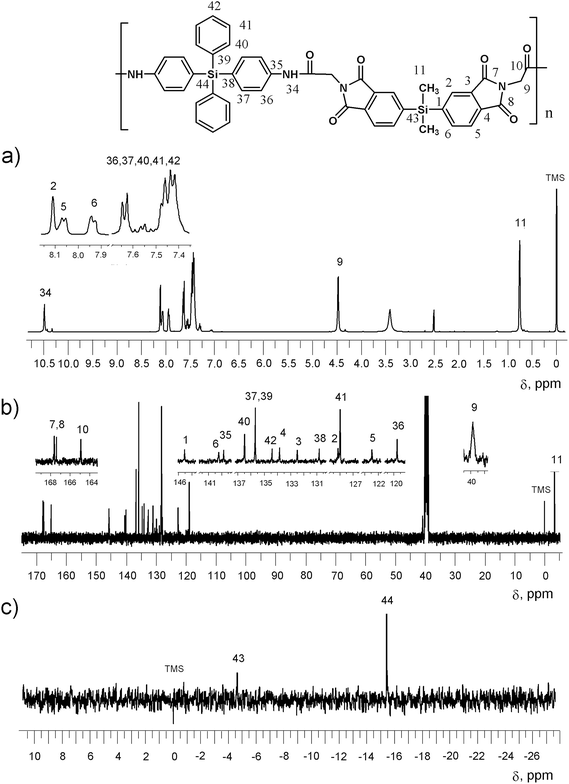
The NMR spectra of the PIAs were very similar to those of the dicarboxylic imido-acids I–III (a–g), but including the signals corresponding of the diamine. The 29Si NMR of the PIAs showed two signals, one corresponding to the diacid moiety at the same values showed before the polymerization, and the second corresponding to the diamine at about −15.3 ppm. This chemical shift to a higher field is due to the effect of the N atoms. Fig. 2 shows the NMR spectra (1H, 13C and 29Si) for PIA-I-a, derived from glycine.
Table 1 shows the results of the solubility test developed for the PIAs. All of them were soluble in aprotic polar solvents like DMSO, DMAc, DMF and NMP, and also in a protic polar solvent usually used for this kind of polymers like m-cresol. PIAs were also soluble in THF and several of them in CHCl3 and acetone, which is an important advance in the processability of this kind of polymers. The improvement of the solubility can be due to the polarity of the C–Si bond and to the presence of the side groups provided by the amino acids moieties. In fact, PIAs including glycine and p-aminobenzoic acid as amino acids, without a side chain, were not soluble in CHCl3 and acetone, with the exception of PIA-III-g which showed solubility in this last solvent. Probably this effect can be explain due to the steric effect of the aromatic ring bonded to the two Si atoms, which promote a great free volume between the polymeric chains with low packing forces between them. So, the incorporation of solvent to the polymer matrix could be favored.
| Series | DMSO | NMP | DMF | DMAc | m-Cresol | THF | CHCl3 | Acetone |
|---|---|---|---|---|---|---|---|---|
| a + soluble at room temperature, ++ soluble at 40 °C, – insoluble at room temperature. | ||||||||
| PIA-I-a | + | + | + | + | + | + | − | − |
| PIA-I-b | + | + | + | + | + | + | + | ++ |
| PIA-I-c | + | + | + | + | + | + | + | + |
| PIA-I-d | + | + | + | + | + | + | + | + |
| PIA-I-e | + | + | + | + | + | + | + | + |
| PIA-I-f | + | + | + | + | + | + | + | + |
| PIA-I-g | + | + | + | + | + | + | − | − |
| PIA-II-a | + | + | + | + | + | + | − | − |
| PIA-II-b | + | + | + | + | + | + | + | + |
| PIA-II-c | + | + | + | + | + | + | + | + |
| PIA-II-d | + | + | + | + | + | + | + | + |
| PIA-II-e | + | + | + | + | + | + | + | + |
| PIA-II-f | + | + | + | + | + | + | + | + |
| PIA-II-g | + | + | + | + | + | + | − | − |
| PIA-III-a | + | + | + | + | + | + | − | − |
| PIA-III-b | + | + | + | + | + | + | + | + |
| PIA-III-c | + | + | + | + | + | + | + | + |
| PIA-III-d | + | + | + | + | + | + | + | + |
| PIA-III-e | + | + | + | + | + | + | + | + |
| PIA-III-f | + | + | + | + | + | + | + | + |
| PIA-III-g | + | + | + | + | + | + | + | − |
Table 2 shows the yields obtained for the polymerization process, which were almost quantitative (90–99%) and indicative of the efficiency of the Yamazaki' polyimidation process.52,53
| Series | Yield (%) | [α]Da (°) | ηinhb (dL g−1) | Tg (°C) | TDT (°C) |
|---|---|---|---|---|---|
| a In NMP at 25 °C.b Inherent viscosity, in NMP at 25 °C (c = 0.5 g dL−1). | |||||
| PIA-I-a | 99 | — | 0.18 | 240 | 443 |
| PIA-I-b | 98 | −20 | 0.13 | 220 | 419 |
| PIA-I-c | 98 | −60 | 0.11 | 200 | 432 |
| PIA-I-d | 90 | −40 | 0.13 | 195 | 431 |
| PIA-I-e | 97 | +20 | 0.14 | 175 | 429 |
| PIA-I-f | 99 | +20 | 0.12 | 165 | 377 |
| PIA-I-g | 99 | — | 0.17 | 260 | 493 |
| PIA-II-a | 99 | — | 0.13 | 210 | 444 |
| PIA-II-b | 96 | +20 | 0.10 | 184 | 419 |
| PIA-II-c | 98 | +40 | 0.10 | 201 | 412 |
| PIA-II-d | 98 | +20 | 0.10 | 194 | 436 |
| PIA-II-e | 99 | +20 | 0.10 | 158 | 429 |
| PIA-II-f | 94 | +20 | 0.10 | 180 | 400 |
| PIA-II-g | 99 | — | 0.10 | 228 | 508 |
| PIA-III-a | 99 | — | 0.12 | 185 | 442 |
| PIA-III-b | 94 | +40 | 0.10 | 236 | 406 |
| PIA-III-c | 92 | +20 | 0.10 | 176 | 442 |
| PIA-III-d | 93 | +100 | 0.10 | 204 | 398 |
| PIA-III-e | 96 | +60 | 0.11 | 165 | 426 |
| PIA-III-f | 92 | +60 | 0.10 | 170 | 412 |
| PIA-III-g | 93 | — | 0.10 | 234 | 490 |
Table 2 also shows the inherent viscosity values obtained in NMP at 25 °C (c = 0.5 g dL−1). The obtained values were low, probably corresponding to oligomeric species, especially those derived from dicarboxylic imido-acids II-(a–g) and III-(a–g), which contain aromatic rings bonded to the Si atom. Probably this structural detail promotes a partial insolubility of the PIAs in the reaction media, which increases when the aromatic content is increased. PIAs derived from the dicarboxylic imido-acids I-(a–g) without aromatic rings bonded to the Si atom showed a little increment in their ηinh value, especially PIA-I-a and PIA-I-b, for which the amino acids moieties have not side chains.
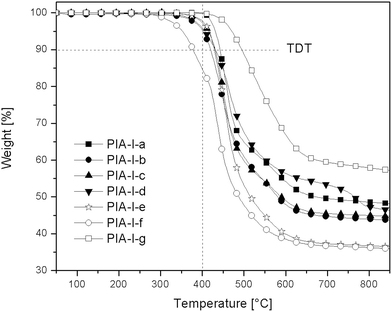
The results obtained for the thermal properties, in particular, glass transition temperature Tg, and thermal degradation temperature TDT, are summarized in Table 2.
In general the Tg values of the synthesized PIAs were not so high taking in account that aromatic poly(amides) and poly(imides) normally show higher values of this parameter. The effect of the amino acid moiety including a C sp3 in the main chain can be responsible of these values.
It is possible to see in the series of PIAs derived from the dicarboxylic imido-acids I-(a–g), that the values decrease when the side chain of the amino acid moiety is increased. This fact can be due to the higher rotational freedom of the chains promoted by the higher separation between them. This effect implies that the mobility of the chains is higher. Also contribute to this effect the two methyl groups bonded to the Si atom of the acid residue, without π–π aromatic interactions.
PIAs derived from the three dicarboxylic imido-acids with p-aminobenzoic acid (PIA-I-g, PIA-II-g and PIA-III-g), showed the higher Tg values in each series, due to the rigidity of the aromatic ring of this amino acid residue.
Respect to the values obtained for the PIAs derived from dicarboxylic imido-acids II, also there are a similar tendency but with two exceptions, PIA-II-b derived from L-alanine which showed a low value and PIA-II-f derived from L-isoleucine which showed a high value, we do not have an adequate explanation for these results.
Tg values obtained for those PIAs derived from dicarboxylic imido-acids III-(a–g), do not have a clear relationship respect to the structure of the amino acid used. The lower values were obtained for PIA-III-e, PIA-III-f and PIA-III-c, which have L-leucine, L-isoleucine and L-phenylalanine respectively, with the larger side chains. This fact could be explained for an increasing in the internal rotation of the chains.
On the other hand, if we compare the Tg values as a function of the groups bonded to the Si atom of the dicarboxylic imido-acids, there is not a clear tendency. In fact, for those derived from glycine (PIA-I-a, PIA-II-a, PIA-III-a) it was possible to see a decreases of the Tg values when the aromatic content is increased, due to the higher distance between the polymeric chains. The same effect can be seen in PIA-I-c, PIA-II-c and PIA-III-c, having the first two practically the same Tg value.
In the other PIAs groups there was not a clear tendency. Apparently the aromatic interactions between the polymeric chains did not show a great significance, because several of the PIA-III showed the lowest value, compared with those derived from the other dicarboxylic imido-acids but with the same amino acidic residue.
Table 2 shows the thermal decomposition temperatures (TDT10%), taken as the temperature at which the weight loss is 10% and Fig. 3 show the curves obtained for PIAs of series I. In all PIAs, there are two structural differences between them: the groups bonded to the Si atom of the dicarboxylic imido-acids moieties (I–III) and the amino acids residue. According to the classic definition almost all PIAs were thermostables (weight loss lower than 10% at 400 °C), with two exceptions PIA-I-f (TDT10% = 377 °C) derived from imido-diacid If (Fig. 3) with methyl groups bonded to the Si atom and L-isoleucine as amino acid residue, and PIA-III-e which was practically thermostable with a TDT10% of 398 °C.
The most thermostable PIAs were those including p-aminobenzoic acid as amino acid moiety, due to the rigidity of the p-phenylene group in the main chain. Also PIAs including glycine (a) as amino acid moiety (series a) also showed high TDT values due to the lack of side groups.
The other PIAs showed a very similar TDT10% values and there were not a logic sequence, having all of them side groups provided by the amino acids moieties.
If it is compared the TDT10% values as a function of the groups, methyl of phenyl, bonded to the Si atom of the dicarboxylic imido-acids moieties (I, II, III) it is possible to see that these groups have low influence in the TDT10% values. This fact allows to conclude that the amino acidic moieties, as a part of the main chain with a sp3 carbon atom, have higher influence in the degradation process.
Table 3 shows the results obtained for the UV-vis transmittance analyses. A material is considered as transparent when the transmittance is higher than 80% at a wavelength of 400 nm, and depends on the structure and the functional groups present.
| Series | T400 (%) | λT=80%(nm) | λcutoff (nm) |
|---|---|---|---|
| a T400: transmittance at 400 nm. λT=80%:wavelength at 80% of transmittance. λcutoff: cut wavelength. c = 0.5 g dL−1 in NMP at 25 °C. | |||
| PIA-I-a | 86 | 377 | 328 |
| PIA-I-b | 7 | 681 | 261 |
| PIA-I-c | 50 | 499 | 331 |
| PIA-I-d | 81 | 395 | 330 |
| PIA-I-e | 84 | 384 | 329 |
| PIA-I-f | 86 | 382 | 331 |
| PIA-I-g | 81 | 398 | 352 |
| PIA-II-a | 79 | 407 | 328 |
| PIA-II-b | 92 | 363 | 328 |
| PIA-II-c | 17 | 520 | 361 |
| PIA-II-d | 87 | 379 | 329 |
| PIA-II-e | 78 | 403 | 330 |
| PIA-F-f | 50 | 455 | 339 |
| PIA-II-g | 84 | 394 | 354 |
| PIA-III-a | 58 | 452 | 333 |
| PIA-III-b | 10 | 534 | 357 |
| PIA-III-c | 43 | 468 | 344 |
| PIA-III-d | 10 | 490 | 355 |
| PIA-III-e | 1 | 593 | 398 |
| PIA-III-f | 2 | 564 | 387 |
| PIA-III-g | 3 | 514 | 378 |
Fig. 4a shows the curves obtained for PIAs of the series I (R1 = R2 = methyl) where the effect of lateral group of the aminoacidic residue is not clear. Similar results are observer for the polymers from series II and series III. For PIAs of the series II, those including L-alanine, L-valine and p-aminobenzoic moieties, showed values higher than 80%, but the measurements were made in solution because the films were brittles. On the other hand, for PIAs of series I, only those including L-analine and L-phenylalanine do not show transparence at 400 nm.
 | ||
| Fig. 4 UV-visible spectra of (a) PIAs of series I and (b) PIAs derived from N,N′-(4,4′-dimethylsilylenediphthaloyl)-bis-acetic diacid (I-a). | ||
A characteristic of all the synthesized PIAs in this work is the high aromatic content of the repetitive unit. This structural element contributes negatively to the transparence due to the conjugation that present this kind of materials and to the formation of charge transfer complex which reduce this parameter.
Fig. 4b shows that the increase of aromatic content in the imido-diacid residue, reduces the transmittance value. This fact is also observed in Table 3 when PIAs of series III, with the higher aromatic content showed the lower values, and all of them did not show transparence.
Conclusions
Three series of oligomeric poly(imido-amides) (PIAs) containing two Si atoms in the repeating unit of the main chain, derived from seven monomeric imido-diacids containing amino acidic moieties, and an aromatic silylated diamine were synthesized and characterized.Six α-amino acids plus p-aminobenzoic acid and bis(4-aminophenyl)diphenylsilane as the diamine were used in the synthetic process. PIAs were obtained with almost quantitative yields but with low ηinh values, concluding that PIAs were of oligomeric nature.
PIAs were soluble in aprotic polar solvents, and also in other common solvents like THF, acetone and CHCl3, due to the inclusion of Si atoms and to the presence of the aliphatic amino acidic residues.
Tg values showed a decrease when the aliphatic content of the amino acid residue increased, due to a higher chain separation and higher free rotation of them. PIAs derived from amino acids without side chains, like glycine and p-aminobenzoic acid showed the higher Tg values due to an increase of the molecular rigidity.
Almost all the PIAs were thermally stable, being the most stables those including p-aminobenzoic as amino acidic residue. The groups bonded to the Si atom of the dicarboxylic imido-acids I, II and III, do not showed an important influence.
Finally, an increase of the aromatic content reduced the transparence values, and those containing only aromatic rings bonded to the Si atoms (PIA-III-(a–g)) did not show UV-vis transparence, due to the high aromatic content of the structure.
Acknowledgements
The author acknowledge the financial support by Fondo Nacional de Investigación Científica y Tecnológica (FONDECYT) through grant 1100015. P.A.O. acknowledges the fellowship of CONICYT (Comisión Nacional de Investigación Científica y Tecnológica).References
- J. K. Fink, High Performance Polymers, William Andrew Inc., Nueva York, USA, 2008 Search PubMed.
- S. Mallakpour and E. Kowsari, Polym. Adv. Technol., 2005, 16, 732–737 CrossRef CAS.
- D. Liaw and B. Liaw, Polymer, 2001, 42, 839–845 CrossRef CAS.
- S. Nakata and J. J. Brisson, J. Polym. Sci., Part A: Polym. Chem., 1997, 35, 2379–2386 CrossRef CAS.
- D. J. Liaw, P. N. Hsu and B. Y. Liaw, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 63–70 CrossRef CAS.
- D. J. Liaw, B. Y. Liaw and C. M. Yang, Macromol. Chem. Phys., 2001, 202, 1866–1872 CrossRef CAS.
- E. Ferrero, J. F. Espeso, J. G. de la Campa, J. de Abajo and A. E. Lozano, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 3711–3724 CrossRef CAS.
- V. Ayala, E. M. Maya, J. M. García, J. G. de la Campa, A. E. Lozano and J. de Abajo, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 112–121 CrossRef CAS.
- S. H. Hsiao, C. P. Yang, C. W. Chen and G. S. Liou, Eur. Polym. J., 2005, 41, 511–517 CrossRef CAS PubMed.
- I. Sava and M. Bruma, Macromol. Symp., 2006, 239, 36–42 CrossRef CAS.
- S. Mehdipour-Ataei, Eur. Polym. J., 2005, 41, 65–71 CrossRef CAS PubMed.
- S. Mallakpour and M. Kolahdoozan, Iran. Polym. J., 2006, 15, 307–315 CAS.
- F. Sanda and T. Endo, Macromol. Chem. Phys., 1999, 200, 2651–2661 CrossRef CAS.
- R. Katsarava, Macromol. Symp., 2003, 199, 419–430 CrossRef CAS.
- T. W. Baughman and K. B. Wagener, Adv. Polym. Sci., 2005, 176, 1 CrossRef CAS.
- S. Mallakpour and F. Rafiemanzelat, J. Appl. Polym. Sci., 2005, 98, 1781–1792 CrossRef CAS.
- S. Mallakpour and E. Kowsari, J. Appl. Polym. Sci., 2006, 99, 1038–1044 CrossRef CAS.
- E. Abdul Rahim, F. Sanda and T. Masuda, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 810 CrossRef CAS.
- S. Mallakpour and E. Kowsari, Polym. Eng. Sci., 2006, 46, 558–565 CAS.
- S. Mallakpour and M. H. Shahmohammadi, Iran. Polym. J., 2005, 14, 974–981 CAS.
- M. Bruma and B. Schulz, J. Macromol. Sci., Part C: Polym. Rev., 2001, 41, 1–40 CrossRef PubMed.
- S. B. Speck, J. Org. Chem., 1953, 18, 1689–1700 CrossRef CAS.
- M. Bruma, I. Sava, F. Mercer, V. N. Reddy, T. Köpnick, B. Stller and B. Schulz, Polym. Adv. Technol., 1998, 9, 752–758 CrossRef CAS.
- M. Bruma, B. Schulz, T. Köpnick and J. Robinson, High Perfom. Polym., 2000, 12, 429–443 CrossRef CAS.
- B. Schulz, E. Hamciuc, T. Köpnick, Y. Kaminorz and M. Bruma, Macromol. Symp., 2003, 199, 391–400 CrossRef CAS.
- E. Hamciuc, C. Hamciuc, M. Bruma and B. Schulz, Eur. Polym. J., 2005, 41, 2989–2997 CrossRef CAS PubMed.
- N. D. Ghatge and J. Y. Jadhav, J. Polym. Sci., Polym. Chem. Ed., 1983, 21, 3055–3061 CrossRef CAS.
- N. D. Ghatge and J. Y. Jadhav, J. Polym. Sci., Polym. Chem. Ed., 1984, 22, 1565–1572 CrossRef CAS.
- J. Y. Jadhav, N. N. Chavan and N. D. Ghatge, Eur. Polym. J., 1984, 20, 1009–1011 CrossRef CAS.
- S. F. Thames and K. G. Malone, J. Polym. Sci., Part A: Polym. Chem., 1993, 31, 521–526 CrossRef CAS.
- J. Zhang, Q. Sun and X. Hou, Macromolecules, 1993, 26, 7176–7181 CrossRef CAS.
- M. D. Joshi, A. Sarkar, O. S. Yemul, P. P. Wadgaonkar, S. V. Lonikar and N. N. Maldar, J. Appl. Polym. Sci., 1997, 64, 1329–1335 CrossRef CAS.
- I. Sava and E. Hamciuc, Rev. Roum. Chim., 2007, 52, 127–133 CAS.
- I. Sava, M. Bruma, B. Schulz and T. Kopnick, High Perform. Polym., 2005, 17, 483–495 CrossRef CAS PubMed.
- L. H. Tagle, C. A. Terraza, A. Tundidor-Camba and F. A. Lara, Polym. Bull., 2014, 71, 1031–1042 CrossRef CAS PubMed.
- L. H. Tagle, C. A. Terraza, A. Tundidor-Camba and J. Soto-Salamanca, Polym. Bull., 2014, 71, 287–300 CrossRef CAS PubMed.
- L. H. Tagle, C. A. Terraza, A. Tundidor-Camba and F. A. Lara, J. Chil. Chem. Soc., 2013, 58, 2114–2116 CrossRef.
- L. H. Tagle, C. A. Terraza, A. Leiva and D. Coll, High Perform. Polym., 2012, 24, 77–84 CrossRef CAS PubMed.
- L. H. Tagle, C. A. Terraza, P. A. Ortiz, M. J. Rodríguez, A. Tundidor-Camba, A. Leiva, C. González-Henríquez, A. L. Cabrera, U. G. Volkmann and E. Ramos-Moore, J. Macromol. Sci., Part A: Pure Appl.Chem., 2012, 49, 562–570 CrossRef CAS.
- C. M. González Henríquez, L. H. Tagle, C. A. Terraza, A. Barriga González, U. G. Volkmann, A. L. Cabrera, E. Ramos and M. Pavez, Polym. Int., 2012, 61, 197–204 CrossRef.
- L. H. Tagle, C. A. Terraza, H. Villagra and A. Tundidor-Camba, Polym. Bull., 2011, 67, 1799–1807 CrossRef CAS.
- L. H. Tagle, C. A. Terraza, A. Leiva, N. Yazigi and L. López, J. Appl. Polym. Sci., 2010, 117, 1526–1534 CAS.
- M. Maienthal, M. Hellmann, C. P. Habe, L. A. Hymo, S. Carpenter and J. Carr, J. Am. Chem. Soc., 1954, 76, 6392–6393 CrossRef CAS.
- H. N. Kovaks, A. D. Delman and B. B. Simms, J. Polym. Sci., Part A-1: Polym. Chem., 1968, 6, 2103–2115 CrossRef.
- J. Zhang, Q. Sun and X. Hou, Macromolecules, 1993, 26, 7176–7181 CrossRef CAS.
- L. H. Tagle, F. R. Diaz, J. C. Vega and P. Valenzuela, Eur. Polym. J., 2003, 39, 407–410 CrossRef CAS.
- L. H. Tagle, C. A. Terraza, A. Leiva and P. Valenzuela, J. Appl. Polym. Sci., 2006, 102, 2768–2776 CrossRef CAS.
- S. Mallakpour and E. Kowsari, J. Appl. Polym. Sci., 2005, 96, 435–442 CrossRef CAS.
- K. Faghihi and M. Gholizadeh, Turk. J. Chem., 2009, 33, 87–97 CAS.
- E. Hamciuc, M. Bruma, B. Schulz and T. Köpnick, High Perform. Polym., 2003, 15, 347–359 CrossRef CAS PubMed , and the references therein.
- J. R. Pratt, W. D. Massey, F. M. Pinkerton and S. F. Thames, J. Org. Chem., 1975, 40, 1090–1094 CrossRef CAS.
- N. Yamazaki, N. Matsumoto and F. Higashi, J. Polym. Sci., Polym. Chem. Ed., 1975, 13, 1373–1380 CrossRef CAS.
- A. Tundidor-Camba, C. A. Terraza, L. H. Tagle and D. Coll, J. Appl. Polym. Sci., 2011, 120, 2381–2389 CrossRef CAS.
- C. A. Terraza, L. H. Tagle, F. Concha and L. Poblete, Des. Monomers Polym., 2007, 10, 253–261 CrossRef CAS PubMed.
- A. Factor and P. T. Engen, J. Polym. Sci., Part A: Polym. Chem., 1993, 31, 2231–2236 CrossRef CAS.
- L. H. Tagle, C. A. Terraza, W. Ahlers and C. Vera, J. Chil. Chem. Soc., 2005, 50, 535–539 CAS.
- W. Uhlig, Prog. Polym. Sci., 2002, 27, 255–305 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |