Induction coil heater prepared highly fluorescent carbon dots as invisible ink and explosive sensor†
Abstract
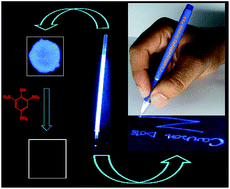
We report a new and facile method for the synthesis of highly fluorescent carbon dots (Cdots) using a commercially available induction coil heater. An aqueous solution of citric acid and a diamine compound was heated at 100 °C for 12–15 min, upon which the Cdots were produced. The Cdots, with an average size of less than 5 nm (produced when ethylenediamine was used), emitted a blue light with a high quantum yield when excited under UV light. The quantum yield was dependent on the nature of diamine and was as high as 73.5% for ethylenediamine. The as-prepared Cdots could be easily converted into a gel by mixing with chitosan biopolymer. The gel could be used for filling up the refill of a ball-point pen and can be used for UV-active marking, for sensing of explosive compounds (such as picric acid and 2,4-dinitrophenol) with high efficiency and for other fluorescence based applications. The use of a commercial induction coil heater, scalability and high chance of commercial viability make the method particularly appealing.

 Please wait while we load your content...
Please wait while we load your content...