Enhanced UV detection performance using a Cu-doped ZnO nanorod array film†
Abstract
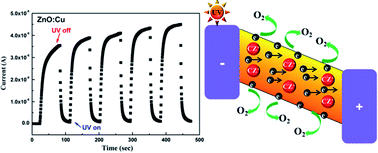
A compact Cu-doped ZnO nanorod (NR) array film was synthesized by a facile hydrothermal method and post-annealing process. The obtained ZnO NR array film-based UV photodetectors exhibit not only enhanced UV sensitivity but also faster reset time compared to undoped ZnO NR samples, which are attributed to the trapping and de-trapping of electrons by Cu-related defects.

 Please wait while we load your content...
Please wait while we load your content...