Hydrogen evolution inhibition with diethylenetriamine modification of activated carbon for a lead-acid battery
Abstract
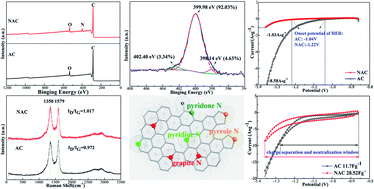
A novel idea to inhibit the hydrogen evolution in activated carbon (AC) application in a lead-acid battery has been presented in this paper. Nitrogen group-enriched AC (NAC, mainly exists as pyrrole N) was prepared. Electrochemical measurements demonstrate that the hydrogen evolution reaction (HER) is markedly inhibited as the HER impedance increases significantly. What's more, the specific capacitance value of NAC is 142.5% higher than AC since the working window is extended. The use of NAC, instead of AC in an UltraBattery, can inhibit hydrogen evolution, and improve the battery's charge acceptance and charge retention ability.


 Please wait while we load your content...
Please wait while we load your content...