Improving thermal conductivity and decreasing supercooling of paraffin phase change materials by n-octadecylamine-functionalized multi-walled carbon nanotubes†
Abstract
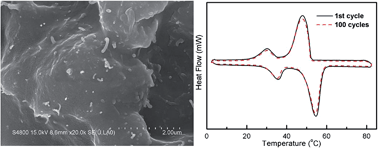
Improving thermal conductivity and decreasing supercooling are essential for the utilization of paraffin phase change materials (PCMs). In this work, n-octadecylamine-functionalized multi-walled carbon nanotubes (f-MWCNTs) are obtained through a simple method of carboxylation of the MWCNTs with mixed acids of H2SO4 and HNO3 and then salt-forming reaction with n-octadecylamine. The paraffin/f-MWCNTs (paraffin/f-MWCNTs) composite PCMs are fabricated by mixing paraffin with f-MWCNTs under ultrasonication at 70 °C. It is found that the f-MWCNTs are homogenously dispersed in a toluene or paraffin matrix due to the existence of long chain alkanes in f-MWCNTs. As a result, the thermal conductivity and heat transfer of the paraffin/f-MWCNTs composite PCMs are significantly enhanced. Moreover, differential scanning calorimetry (DSC) analysis indicates that the incorporation of f-MWCNTs reduces the supercooling of paraffin, mainly due to the well-dispersed f-MWCNTs serving as nuclei to promote the heterogeneous nucleation and crystallization process of paraffin.

 Please wait while we load your content...
Please wait while we load your content...