Variations of interlayer spacing in carbon nanotubes
Oxana V. Kharissova
and
Boris I. Kharisov
*
Universidad Autónoma de Nuevo León, Monterrey, México. E-mail: bkhariss@hotmail.com
First published on 19th June 2014
Abstract
The analysis of previous classic and recent reports on the interlayer distances in MWCNTs is described in this review. Simulations on interlayer spacing, applications of Raman spectroscopy, X-ray and neutron diffraction methods, influence of synthesis methods, heat and radiation (gamma-rays, electron and ion beams) treatments are discussed, as well as the polygonization and intercalation of CNTs. It is shown that the spacing values of DWCNTs and MWCNTs vary from 0.27 up to 0.42 nm. The most common values are in the range of 0.32–0.35 nm and do not strongly depend on the synthesis method. Diameter of CNTs and the symmetry of layers influence the interwall spacing.
Introduction
Carbon nanotubes (CNTs),1,2 among other carbon allotropes in nanotechnology,3 are very well studied after publication of thousands of experimental articles, reviews, books and chapters. Interlayer distance/spacing, an important property of multi-wall CNTs, was determined much earlier,4,5 in particular by Dresselhaus,6 who observed that the interlayer distance ranges from 0.342 to 0.375 nm, and that it is a function of curvature and the number of layers/shells comprising the tube. However, in the last 15 years several reports have appeared periodically, sometimes contradicting these reports, on slight variations between interlayer distances. We believe that this topic has not yet lost its importance because the carbon nanotubes possess enormous applications, where properties of their nanocomposites and nanomaterials could depend on interspacing distances. In this short review, we present a generalization of reports during this period, paying attention to the main reasons for interlayer spacing variations.Classic definitions
It is well-known that the CNTs form two structurally distinct classes. The first to be discovered, multi-wall CNTs (MWNTs), exhibit, in particular, a Russian doll-like structure of nested concentric tubes.7 The interlayer spacing in 3D-crystalline graphite is 0.335 nm, suggesting a similar weak interaction between individual shells in MWNTs (Fig. 1).8 When a nanotube contains only two layers, it is referenced as a double-walled carbon nanotube (DWNT). Interlayer spacing of DWNTs is not a constant and ranges from 0.34 nm to 0.41 nm.9 Mechanical properties of CNTs are closely related to those of graphite. The outer diameter of MWCNTs starts from 2.5 nm, while for single-wall CNTs (SWCNTs) this value ranges from 0.6 to 2.4 nm.10,11 Stiff sp2-hybridized in-plane σ-bonds, 1.42 Å long, give them an exceptionally high Young's modulus, while out-of-plane π-bonds, which are responsible for the main features of electronic properties, govern the weak van der Waals interlayer cohesion. The different shells of MWNTs interact through van der Waals interaction (in the absence of structural defects) while SWNTs form bundles in which the intertube coupling is also determined by the van der Waals interaction. The combination of strong sp2 bonding and weak van der Waals interaction in these structures lies at the origin of the exceptionally diverse mechanical behavior of nanotubes.In the works of Dresselhaus,12,13 variations in the inter-shell spacing were studied, showing that all the studied tube diameters exhibited an inter-shell spacing (d002) ranging from 0.33 to 0.39 nm, and that (d002) increases as the tube diameter decreases. The simple elastic constant model implies an epitaxy-like growing mechanism for carbon nanotubes. Upon the formation of the first cylinder of a graphene sheet, the diameter of the second cylinder is determined by the energetic equilibrium between the two graphene shells. This process continues until the curvature of the graphene shells becomes negligible such that the elastic energies play less important roles.
Simulations/DFT calculations on interlayer distances
Several simulation studies on interlayer spacing in carbon nanotubes have been carried out using DWCNTs as a model.14,15 In particular, it was indicated that the stability of a DWNT depends only on the interlayer spacing, which reaches an energy minimum when the mean interlayer separation is 0.34 nm, which is independent of the chiralities of the two constituent tubes.16 Atomistic simulations were performed to investigate the torsional behavior of abnormal DWCNTs (Fig. 2) with an interlayer distance of less than 0.34 nm (Table 1) and carbon nanowires (CNWs) made of linear carbon-atom chain (C-chain) encapsulated inside single-walled carbon nanotubes (SWCNTs) subject to torsional motion.17 These simulations indicated that the effect of van der Waals (vdW) interaction is more significant for abnormal DWCNTs than for normal DWCNTs. The critical torsional moments of abnormal DWCNTs are considerably enhanced compared with those of corresponding normal DWCNTs. It is worth noting that the critical torsional moment does not always increase with a decrease in the interlayer distance of DWCNTs. | ||
| Fig. 2 The primary buckling morphologies of DWCNTs: (a) ((5, 5), (10, 10)) DWCNT; (b) ((6, 6), (10, 10)) DWCNT and (c) ((7, 7), (10, 10)) DWCNT. | ||
| DWCNTs | Inner tube radius (nm) | Outer tube radius (nm) | Interlayer distance (nm) |
|---|---|---|---|
| ((4, 0), (13, 0)) | 0.157 | 0.509 | 0.352 |
| ((5, 0), (13, 0)) | 0.196 | 0.509 | 0.313 |
| ((6, 0), (13, 0)) | 0.235 | 0.509 | 0.274 |
The equilibrium structures of DWNT, as well as the interwall interaction energies of DWNT, were computed using a local density approximation within DFT theory with periodic boundary conditions and Gaussian-type orbitals.18 The interwall distances d, translational lengths td of the unit cell, relative differences tδ = (ts2 − ts1)/td, and Young's moduli Ed of DWNT are presented in Table 2. For the armchair (n, n)@(n+5, n+5) and zigzag (9, 0)@(18, 0) DWNT, the interwall distance is closer to the interlayer separation in graphite, and the calculated values of the energy Uint are comparable with the interwall interaction energy of 35–40 meV per atom obtained using the Lennard-Jones potential and the experimental value of the interlayer interaction in graphite.
| Nanotube | d, Å | td | tδ | Ed | Uint |
|---|---|---|---|---|---|
| (4, 4)@(10, 10) | 4.011 | 2.4444 | 0.00317 | 0.992 ± 0.002 | 13.29 |
| (5, 5)@(11, 11) | 4.017 | 2.4424 | 0.00061 | 0.989 ± 0.003 | 13.36 |
| (6, 6)@(12, 12) | 4.021 | 2.4421 | 0.00020 | 1.005 ± 0.003 | 13.67 |
| (5, 5)@(10, 10) | 3.344 | 2.4425 | 0.00058 | 1.085 ± 0.004 | 23.83 |
| (6, 6)@(11, 11) | 3.350 | 2.4421 | 0.00019 | 1.106 ± 0.003 | 24.09 |
| (7, 7)@(12, 12) | 3.353 | 2.4421 | 0.00013 | 1.106 ± 0.003 | 24.60 |
| (9, 0)@(18, 0) | 3.478 | 4.2315 | 0.00116 | 1.049 ± 0.003 | 24.26 |
| (10, 0)@(20, 0) | 3.875 | 4.2305 | 0.00091 | 1.016 ± 0.002 | 16.76 |
Large-scale quasi-continuum simulations were performed to determine the stable cross-sectional configurations of free-standing MWCNTs {armchair (AC), zigzag (ZG), and chiral (CH) MWCNTs} (Fig. 3 and 4).19 It was observed that at an interwall spacing larger than the equilibrium distance set by the interwall van der Waals (vdW) interactions, the initial circular cross-sections of the MWCNTs are transformed into symmetric polygonal shapes or asymmetric water-drop-like shapes. The simulations also showed that removing several innermost walls causes an even more drastic cross-sectional polygonization of the MWCNTs. It was also shown that the symmetry of the layers of the multi-wall tubes strongly affects the interwall interaction.20 The strongest interaction was observed for the commensurate tubes with achiral walls. On the other side, the tubes with chiral walls interacted negligibly.
 | ||
| Fig. 3 Cross-sectional views of the relaxed MWCNTs. From left to right on each row, the wall numbers are 5, 10, 15, 20, and 25. Top AC MWCNTs; middle ZG MWCNTs; bottom CH MWCNTs. | ||
 | ||
| Fig. 4 The inter-wall spacing of the relaxed ZG MWCNTs shown in the previous Figure {from (f) to (j)}. | ||
The effect of the intertube van der Waals interaction on the stability of pristine and covalently functionalized CNTs under axial compression was investigated21 using molecular mechanics simulations. After regulating the number of inner layers of the armchair four-wall (5, 5)@(10, 10)@(15, 15)@(20, 20) and zigzag four-wall (6, 0)@(15, 0)@(24, 0)@(33, 0) carbon nanotubes, the critical buckling strains of the corresponding tubes were calculated. It was emphasized that the equilibrium interlayer space is 0.337 nm and 0.350 nm for the pristine armchair and zigzag tubes, respectively,22 because of different chirality structures. The equilibrium space for any two graphene layers is 0.34 nm. Therefore, there exists an intertube vdW repulsive interaction in the armchair multi-wall tubes and an intertube attractive interaction in the zigzag multi-wall tubes, even when no compression strain is applied. When compression strain is applied, both the Poisson effect and the domino effect, resulting from the collapse, contribute to the repulsive interaction, significantly enhancing the stability of the armchair tubes. However, the intertube repulsive interaction occurs only until the intertube space becomes smaller than 0.34 nm. Among zigzag tubes, the deviation of 0.01 nm in the intertube space requires to be compensated by the Poisson effect and the collapse of the outermost tube.
Simulation studies on CNTs can lead to unusual applications. Thus, the DFT calculations of the interaction between nanotube walls were carried out and a new concept was proposed for an electromechanical nanothermometer on the basis of these interactions.23 The energy U of the interaction between two neighboring walls of a DWCNT depends on the coordinates describing the relative position of the walls: the angle φ of relative rotation of the walls around the nanotube axis and the relative displacement z of the walls with respect to this axis. It was demonstrated that a nanothermometer can be used for measuring temperature in spatially localized regions with sizes of several hundred nanometers. Because the measurement of temperature by the nanothermometer under consideration is based on the measurement of conductivity, the nanothermometer can be calibrated using a thermocouple.
Studies by other methods
Raman spectra studies were performed on the DWNTs at different Elaser of excitation.24 The interlayer distance of the DWNTs calculated from radial breathing mode (RBM) was found to vary from 0.335 to 0.42 nm. In addition, a systematic study25 on the diameter dependent spectral features in X-ray diffraction (XRD) and Raman scattering studies of MWCNTs of various diameters in the range of 5–100 nm was carried out. HRTEM imaging revealed a systematic decrease in the interwall separation from 3.8 Å to 3.2 Å as the diameter of nanotubes increased from 5.8 nm to 63.2 nm (Fig. 5). The authors noted that, for very large diameter (80–100 nm) nanotubes, the wall separation first decreases as one moves from the innermost wall to the outer walls and then it again increases near the outermost walls; this may be due to the structural defects at the outer walls of the MWCNT. The analysis of XRD patterns showed an exponential decrease in d002 interlayer spacing with increasing tube diameter, which agrees well with the HRTEM results. The authors believed that the increase in intershell spacing with decreased nanotube diameter results primarily from high curvature and the associated strain in the lower diameter nanotubes. It is likely that high strain in the low diameter tubes causes structural defects in the nanotube walls that may be charged, which causes Coulombic repulsion between the tube walls with charges of the same sign. This would result in a higher d-spacing for the low diameter tubes. On the other hand, in a large diameter MWCNT, the interaction among the walls increases with an increase in the number of walls, and as a result the interwall separation may decrease, as observed experimentally. In addition, in a recent report,26 for raw CNTs, without any admixtures and carbon deposits, average distance between graphene layers (d002) was calculated as follows. The position of the (002) band is connected with an average distance between graphene layers (d002) and can be described as: d002 = λ/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ002, where λ is the wavelength of the X-ray and θ is the Bragg angle of the graphite (002) peak. An average distance between graphene layers was found to be 3.52 Å. An average XRD result was similar but slightly higher (3.62 Å). Indeed, after electronic microscopy methods, X-ray and neutron diffraction measurements of CNTs are classic instrumental techniques to study the interlayer spacing variations in CNTs.27
θ002, where λ is the wavelength of the X-ray and θ is the Bragg angle of the graphite (002) peak. An average distance between graphene layers was found to be 3.52 Å. An average XRD result was similar but slightly higher (3.62 Å). Indeed, after electronic microscopy methods, X-ray and neutron diffraction measurements of CNTs are classic instrumental techniques to study the interlayer spacing variations in CNTs.27
Influence of synthesis method
CVD and microwave plasma-enhanced CVD
The combined growth of CNTs and few-layered graphene sheets (FLGS) by microwave plasma-enhanced chemical vapor deposition was described.28 During the experiment, each position on the samples was exposed to a specific carbon radical concentration. This value was found to be the most important parameter for determining the morphology of the as-grown carbon nanostructures, i.e. either tubular CNTs or plain FLGS. It was shown (Fig. 6) that the flakes on an average consist of 13 atomic layers of graphene because the measured interlayer distance (0.32 nm) corresponds to the tabulated values of graphite. Nickel particles only catalyze the growth of CNTs if the carbon radical concentration is low. A rapid transformation of morphology from tubes to flakes was observed with increasing carbon radical concentration. The flakes formed at the highest carbon radical concentration were identified as FLGS, which were only a few atomic graphene layers thick but up to several micrometers wide. According to an earlier report,29 the results of CVD-synthesized MWCNTs indicated that the interwall coupling in MWNTs is rather weak compared with its parent form, graphite, such that MWNT can be treated as a few decoupled 2D single-wall tubules. The thermal conductivity was found to be low, indicating the existence of substantial defects in the MWNTs prepared by a CVD method. Two facts may be responsible for the weak interwall coupling: the larger interwall distance in MWNTs than the interlayer distance in graphite and the turbostratic stacking of adjacent walls that is unavoidable in rolled-up structures. The interwall distance decreases as the diameter of the tubule increases. At diameters >10 nm, it saturates to ∼0.344 nm, a characteristic interlayer distance in turbostratic stacking. Therefore, the weak interwall coupling in MWNTs is caused by the turbostratic stacking of adjacent walls. | ||
| Fig. 6 TEM micrographs of FLGS show that the flakes on an average consist of 13 atomic layers with a typical interlayer distance of graphite. | ||
Pyrolysis
The unbroken twisted nanotubes, obtained by the pyrolysis of sugar water, exhibited an atomic interlayer distance of ∼0.36 nm (Fig. 7).30 Some of these nanotubes were found to be approximately 10 μm long, which is longer than the length of graphene wall nanotubes fabricated by other pyrolytic template methods.Hydrothermal synthesis and influence of intercalation31
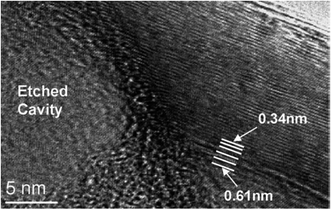
Graphitic carbon nanotubes, synthesized hydrothermally by using an ethylene glycol (C2H6O2) solution in the presence of a Ni catalyst at 730–800 °C under 60–100 MPa pressure, were found to have perfect graphene layers, long and wide internal channels and Ni inclusions in the tips.32 In some of their locations, uniform swelling and intercalation of tube walls, resulting in an almost doubling of the lattice spacing (Fig. 8), were observed (for details on the intercalation of CNTs, see (ref. 33 and 34)). The observed interplanar spacing of 0.61 nm is in agreement with the 0.6–0.7 nm spacing in GO (a similar intercalation of multiwall nanotubes resulting in an increase of lattice spacing up to 0.95 nm was achieved using FeCl3 (ref. 35)). The authors reported that the increased spacing may be because of the penetration of a monolayer of water molecules between graphene sheets. During the growth of a tube, the synthesis fluid, which is a supercritical mixture of CO, CO2, H2O, H2, and CH4, enters the tube. After the closure of the tube and a decrease in temperature, aqueous liquid and gases are trapped inside. Therefore, closed hydrothermal nanotubes, unlike conventional nanotubes produced in vacuum or at ambient pressure, contain water and gases encapsulated under pressure. Considering that the size of oxygen governs that of a water molecule (about 0.3 nm), the increase in spacing because of water penetration should be 0.335 + 0.3 nm = 0.635 nm. This value is comparable with the one measured by authors. In addition, the increase in temperature results in a chemical reaction between the tube and the supercritical fluid and the dissolution of carbon. This reaction leads to the dissolution of the carbon wall (Fig. 9) in the area of the inclusion and, ultimately, puncture of the tube wall and loss of the tube fluid to the microscopic environment.Comparison of various methods
(Interlayer distances between different DWCNTs in their bundles). The DWNTs can be synthesized by different methods such as electric arc discharge, coalescence of C60 peapods, and catalytic chemical vapor deposition (CCVD) using supported or floating catalysts (see ref. 36 and references therein). Depending on these synthesis methods, different values of the intertube spacing have been reported. Large and isolated DWNTs produced by electric arc discharge exhibited an interlayer spacing of 0.39 nm, which is larger than that usually observed for MWNTs (0.34 nm). A similar value, 0.36 nm, was reported for DWNTs synthesized by the coalescence of C60 peapods. Using the CCVD method, an interlayer spacing in the range of 0.34 to 0.41 nm was observed.Influence of heat and irradiation treatments
It was shown37 experimentally that the polygonization (Fig. 10) of multi-walled carbon nanotubes or nanofibers can be induced at sufficiently high heat-treatment temperatures and with sufficiently large diameters. One central finding was the stabilization of polygonal shapes at high temperatures by configuration entropy associated with the creation of the Stone-Wales defects. As a consequence of the polygonization, the interlayer spacing of the MWPNTs contracts to a value distinctly smaller than the established graphene interlayer spacing. The graphene interlayer spacing in multi-walled carbon nanofibers heat treated above ≥2800 K is distinctly smaller than dmin in graphite (0.3354 nm) (Fig. 11). In a related report,38 the thermal expansion of MWCNTs after high-temperature heating (HTT) at 3173 K under a pure argon flow was described, suggesting and confirming that for the as-grown nested MWNTs with high defects, polygonization is preferred for MWNTs greater than 50 nm, while a scroll-like structure is recommendable for MWNTs of diameters less than 50 nm, after heat treatment. XRD-based data on interwall spacing showed that d002 values (in Å) decrease after HTT as follows: (a) average diameter of MWCNTs 10 nm (3.476 → 3.425), 50 nm (3.477 → 3.393), 70 nm (3.478 → 3.391), and 100 nm (3.480 → 3.385). | ||
| Fig. 11 SEM (a) or TEM (b) image showing that the as-grown fibers have circular cross sections (a) and heat-treated fibers are well graphitized (b). (c) Experimentally measured interlayer spacing of the fibers as a function of heating temperature.39 The dotted line marks the spacing of natural graphite. The error bar is within 4 × 10−5 nm, which is smaller than the size of the data points. | ||
The irradiation of MWCNTs with the 800 keV electron beam of a TEM microscope was shown to induce the anisotropic collapse of the nanotube.40 Tight-binding molecular-dynamics simulations of tube response following momentum transfer from large-angle electron-nuclear collisions revealed a strongly anisotropic threshold for atomic displacement. The electron beam preferentially damages the front and back of the nanotube, producing the observed anisotropic collapse perpendicular to the direction of the beam. Collapse accelerates as the graphitic interwall distance of 0.34 nm is approached. Collapse in one portion increases the van der Waals and residual covalent attraction between the nearby opposing sections of the inner wall, possibly inducing a zipper-like closure of the damaged nanotube reminiscent of that anticipated for a mechanically flattened nanotube. In the case of gamma-rays, MWCNTs were irradiated by γ-rays in air and epoxy chloropropane (ECP) with an absorbed dose 200 kGy.41 It was found that MWCNTs showed an opposite behavior in structural change when irradiated in two different media. γ-Ray irradiation decreased the interwall distance of MWCNTs {from 3.44 to 3.42 Å (6%)} and improved their graphitic order in air, while irradiation in ECP increased the interwall distance (from 3.44 to 3.47 Å) of MWCNTs and disordered the structure. The authors explained that γ-rays caused an improvement in the graphitic order of graphite and carbon fibers in air and damaged and reduced the nanotube structure in polar liquid. Because of the great penetrating power of γ-rays, MWCNTs irradiated in air show a significant rearrangement, and the defect concentration can be decreased. As a result, the interwall spacing decreases because the defective graphenes typically have a large interlayer spacing. Another possible mechanism is that irradiation can push one carbon atom out of the graphene plane, and a cross-link between the neighboring graphene layers is then formed. However, in addition to the abovementioned changes, γ-ray irradiation in ECP can shorten tubes, and ECP might be strongly bonded to the dangling bonds of tubes to form grafting chains. The distance of graphene in MWCNTs increases as the defective structure increases. For the case of ion irradiation, the irradiation of a bundle of nanotubes by 100, 250, 500, 750 and 1000 eV Ar ions was simulated.42 It was indicated that the most common defects produced at all energies are vacancies on nanotube walls. The vacancies are metastable and can transform to other defects by saturating dangling bonds and deforming the carbon network of nanotubes. The spatial distribution of the defects proved to be highly non-uniform and has several maximas. These maximas are located at the interface regions between nanotubes in different layers, where atomic density is the highest. It was also demonstrated that ion irradiation results in the formation of intertube covalent bonds mediated by carbon recoils and nanotube lattice distortions due to dangling bond saturation. The number of inter-tube links, as well as the overall damage, linearly grows with the energy of incident ions. In the case of chemical modification/functionalization of CNTs surface, for instance with fluorine atoms,43,44 the interwall spacing of the MWCNT was found to be larger than in the unfluorinated areas.
Conclusions
As it is seen from the earlier reports of 1999–2001 and more recent publications, variations in interlayer/intershell distances (between adjacent graphene layers) from 0.27 to 0.42 nm have been observed for DWCNTs and MWCNTs. The most common values are in the range 0.32–0.35 nm and do not strongly depend on the synthesis method. The diameter of CNTs and symmetry of layers influence the interwall spacing. The interlayer distances could vary upon external treatments (i.e., irradiation or functionalization) and depend on the media (for instance, in air or organic medium). The electronic properties of CNTs can be affected by the variation of interwall spacing. The heat-treatment polygonization of CNTs also influences interwall spacing,45–47 as well as the intercalation. Unusual applications could appear on applying the calculations of the interaction between nanotube walls, for instance, a new concept proposed for an electromechanical nanothermometer. It should be also emphasized that errors in the measurements of interwall spacing by various methods (TEM, XRD, etc.) are significant and can be a reason for variations in the reported data.Finally, while discussing the interlayer spacing in CNTs and graphite, we also need to mention hexagonal boron nitride, which was recently underlined and has a similarity with the abovementioned structures. Specifically, the electrostatic attractions between the oppositely charged atomic centers in adjacent h-BN layers are expected to result in a considerably shorter interlayer distance than that measured for graphite.48 Nevertheless, the interlayer distances in graphite (3.33–3.35 Å) and h-BN (3.30–3.33 Å) are essentially the same, suggesting that electrostatic interactions between partially charged atomic centers, which exist in h-BN and are absent in graphite, have little effect on interlayer bonding. This is consistent with the fact that van der Waals (vdW) forces are responsible for anchoring the h-BN layers at an appropriate interlayer distance rather than electrostatic interactions.
Acknowledgements
The authors are very grateful to Prof. Yuri Gogotsi (Drexel University) for critical revision of this manuscript and highly valuable suggestions.References
- M. Monthioux and V. L. Kuznetsov, Guest Editorial: Who should be given the credit for the discovery of carbon nanotubes?, Carbon, 2006, 44, 1621 CrossRef CAS PubMed
.
- Y. Gogotsi, How safe are nanotubes and other nanofilaments?, Mater. Res. Innovations, 2003, 7, 192–194 CrossRef CAS
.
- Y. Gogotsi, J. A. Libera, N. Kalashnikov and M. Yoshimura, Graphite Polyhedral Crystals, Science, 2000, 290, 317–320 Search PubMed
.
- D. Qian, G. J. Wagner, W. Kam Liu, M. F. Yu and R. S. Ruoff, Mechanics of carbon nanotubes, Appl. Mech. Rev., 2002, 55(6), 495–533 CrossRef
.
- M. Ge and K. Sattler, Bundles of carbon nanotubes generated by vapor-phase growth, Appl. Phys. Lett., 1994, 64(6), 710–711 CrossRef CAS PubMed
.
- C. H. Kiang, M. Endo, P. M. Ajayan, G. Dresselhaus and M. S. Dresselhaus, Size effects in carbon nanotubes, Phys. Rev. Lett., 1998, 81(9), 1869–1872 CrossRef CAS
.
- A. Kis and A. Zettl, Nanomechanics of carbon nanotubes, Philos. Trans. R. Soc., A, 2008, 366, 1591–1611 Search PubMed
.
- M. Endo, T. Hayashi and Y.-A. Kim, Large-scale production of carbon nanotubes and their applications, Pure Appl. Chem., 2006, 78(9), 1703–1713 CrossRef CAS
.
- W. C. Ren, F. Li, J. Chen, S. Bai and H. M. Cheng, Morphology, diameter distribution and Raman scattering measurements of double-walled carbon nanotubes synthesized by catalytic decomposition of methane, Chem. Phys. Lett., 2002, 359, 196–202 CrossRef CAS
.
- G. Cirillo, S. Hampel, U. G. Spizzirri, O. I. Parisi, N. Picci and F. Iemma, Carbon Nanotubes Hybrid Hydrogels in Drug Delivery: a perspective review, BioMed Res. Int., 2014, 2014, 825017 Search PubMed
, 17 pp.
- C. Klumpp, K. Kostarelos, M. Prato and A. Bianco, Functionalized carbon nanotubes as emerging nanovectors for the delivery of therapeutics, Biochim. Biophys. Acta, 2006, 1758, 404–412 CrossRef CAS PubMed
.
- X. Sun, H. J. Zeiger, C.-H. Kiang, M. S. Dresselhaus and M. Endo, Model for interlayer spacing of multiwall carbon nanotubes, Twenty Third Biennial Conference on Carbon, Pennsylvania State University, USA, 1997, http://acs.omnibooksonline.com/data/papers/1997_ii386.pdf, pp. 386–387 Search PubMed.
- P. M. Ajayan and O. Z. Zhou. Applications of Carbon Nanotubes, in Carbon Nanotubes, Topics Appl. Phys., ed. M. S. Dresselhaus, G. Dresselhaus and Ph. Avouris, Springer-Verlag, Berlin Heidelberg, 2001, vol. 80, pp. 391–425 Search PubMed
.
- C. H. Wong and V. Vijayaraghavan, Nanomechanics of Nonideal Single- and Double-Walled Carbon Nanotubes, J. Nanomater., 2012, 2012, 490872 Search PubMed
, 9 pp.
- H. W. Zhang, L. Wang and J. B. Wang, Computer simulation of buckling behavior of double-walled carbon nanotubes with abnormal interlayer distances, Comput. Mater. Sci., 2007, 39(3), 664–672 CrossRef CAS PubMed
.
- F. Li, S. G. Chou, W. Ren, J. A. Gardecki, G. R. Harrison, A. K. Swan, M. S. Unlu, B. B. Goldberg, H.-M. Cheng and M. S. Dresselhaus, Identification of the constituents of double-walled carbon nanotubes using Raman spectra taken with different laser-excitation energies, J. Mater. Res., 2003, 18(5), 1251–1258 CrossRef CAS
.
- H.-Y. Song, L.-F. Li and F. Feng, Torsional behaviour of carbon nanotubes with abnormal interlayer distances, J. Phys. D: Appl. Phys., 2009, 42(055414), 5, DOI:10.1088/0022-3727/42/5/055414
.
- E. Bichoutskaia, M. I. Heggie, A. M. Popov and Y. E. Lozovik, Interwall interaction and elastic properties of carbon nanotubes, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 045435 CrossRef
.
- X. Huang, W. Liang and S. Zhang, Radial corrugations of multi-walled
carbon nanotubes driven by inter-wall nonbonding interactions, Nanoscale Res. Lett., 2011, 6(53), 6 Search PubMed
.
- T. Vukovic, M. Damnjanovic and I. Milosevic, Interaction between layers of the multi-wall carbon nanotubes, Physica E, 2003, 16(2), 259–268 CrossRef CAS
.
- Y. D. Kuang, S. Q. Shi, P. K. L. Chan and C. Y. Chen, The effect of intertube van der Waals interaction on the stability of pristine and functionalized carbon nanotubes under compression, Nanotechnology, 2010, 21, 125704, DOI:10.1088/0957-4484/21/12/125704
, 6 pp.
- W. S. Su, T. C. Leung and C. T. Chan, Work function of single-walled and multi-walled carbon nanotubes: first-principles study, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 235413 CrossRef
.
- A. M. Popov, Y. E. Lozovik, E. Bichoutskaia, G. S. Ivanchenko, N. G. Lebedev and E. K. Krivorotov, An Electromechanical Nanothermometer Based on Thermal Vibrations of Carbon Nanotube Walls, Phys. Solid State, 2009, 51(6), 1306–1314 CrossRef CAS
.
- J. Wei, B. Jiang, X. Zhang, H. Zhu and D. Wu, Raman study on double-walled carbon nanotubes, Chem. Phys. Lett., 2003, 376, 753–757 CrossRef CAS
.
- D. K. Singh, P. K. Iyer and P. K. Giri, Diameter dependence of interwall separation and strain in multiwalled carbon nanotubes probed by X-ray diffraction and Raman scattering studies, Diamond Relat. Mater., 2010, 19, 1281–1288 CrossRef CAS PubMed
.
- M. Pawlyta, D. Łukowiec and A. D. Dobrzańska-Danikiewicz, Characterisation of carbon nanotubes decorated with platinum nanoparticles, Journal of Achievements in Materials and Manufacturing Engineering, 2012, 53(2), 67–75 Search PubMed
.
- J. Dore, A. Burian and S. Tomita, Structural studies of carbon nanotubes and related materials by neutron and X-ray diffraction, Acta Phys. Pol., 2000, 98(5), 495–504 CAS
.
- A. Malesevic, R. Kemps, L. Zhang, R. Erni, G. Van Tendeloo and C. Van Haesendonck, A versatile plasma tool for the synthesis of carbon nanotubes and few-layer graphene sheets, J. Optoelectron. Adv. Mater., 2008, 10(8), 2052–2055 CAS
.
- W. Yi, L. Lu, Z. W. Pan and S. S. Xie, Linear specific heat of carbon nanotubes, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59(14), R9015 Search PubMed
.
- V. Y. Butko, A. V. Fokina, V. N. Nevedomskya and Y. A. Kumzerov, A template method for carbon nanotube production from sugar water, Cornell University Library, 2013, arXiv:1309.3316, http://arxiv.org/abs/1309.3316 Search PubMed
.
- H. Ye, N. Naguib and Y. Gogotsi, TEM Study of Water in Carbon Nanotubes, JEOL News, 2004, 39(2), 2–7 Search PubMed
.
- Y. Gogotsi, N. Naguib and J. A. Libera, In situ chemical experiments in carbon nanotubes, Chem. Phys. Lett., 2002, 365, 354–360 CrossRef CAS
.
- O. Zhou, B. Gao, C. Bower, L. Fleming and H. Shimoda, Structure and Electrochemical Properties of Carbon Nanotube Intercalation Compounds, Mol. Cryst. Liq. Cryst., 2000, 340, 541–546 CrossRef CAS
.
- O. Zhou, H. Shimoda, B. Gao, S. Oh, L. Fleming and G. Yue, Materials Science of Carbon Nanotubes: Fabrication, Integration, and Properties of Macroscopic Structures of Carbon Nanotubes, Acc. Chem. Res., 2002, 35, 1045–1053 Search PubMed
.
- V. Z. Mordkovich, M. Baxendale, M. Yudasaka, R. Kikuchi, S. Yoshimura, J.-Y. Dai and R. P. H. Chang, in Supercarbon: Synthesis, Properties and Applications, ed. S. Yoshimura and R. P. H. Chang, Springer, Berlin, 1998 Search PubMed
.
- J.-F. Colomer, L. Henrard, G. Van Tendeloo, A. Lucas and P. Lambin, Study of the packing of double-walled carbon nanotubes into bundles by transmission electron microscopy and electron diffraction, J. Mater. Chem., 2004, 14, 603–606 RSC
.
- M. Yoon, J. Howe, G. Tibbets, G. Eres and Z. Zhang, Polygonization and anomalous graphene interlayer spacing of multi-walled carbon nanofibers, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75(165402), 6 CrossRef PubMed
.
- F. Y. Wu and H. M. Cheng, Structure and thermal expansion of multi-walled carbon nanotubes before and after high temperature treatment, J. Phys. D: Appl. Phys., 2005, 38, 4302–4307 Search PubMed
.
- J. Y. Howe, G. G. Tibbetts, C. Kwag and M. L. Lake, Heat treating carbon nanofibers for optimal composite performance, J. Mater. Res., 2006, 21, 2646–2652 Search PubMed
.
- V. H. Crespi, N. G. Chopra, M. L. Cohen, A. Zettl and S. G. Louie, Anisotropic electron-beam damage and the collapse of carbon nanotubes, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54(8), 5927–5931 CrossRef CAS
.
- Z. Xu, L. Chen, L. Liu, X. Wu and L. Chen, Structural changes in multi-walled carbon nanotubes caused by γ-ray irradiation, Carbon, 2011, 49, 339–351 CrossRef PubMed
.
- E. Salonen, A. V. Krasheninnikov and K. Nordlund, Ion-irradiation-induced defects in bundles of carbon nanotubes, Nucl. Instrum. Methods Phys. Res., Sect. B, 2002, 193, 603–608 Search PubMed
.
- K. H. An, J. G. Heo, K. G. Jeon, D. J. Bae, C. Jo, C. W. Yang, C.-Y. Park and Y. H. Lee, X-ray photoemission spectroscopy study of fluorinated single-walled carbon nanotubes, Appl. Phys. Lett., 2002, 2002(80), 4235–4237 Search PubMed
.
- K. A. Wepasnick, B. A. Smith, J. L. Bitter and D. Howard Fairbrother, Chemical and structural characterization of carbon nanotube surfaces, Anal. Bioanal. Chem., 2010, 396(3), 1003–1014 Search PubMed
.
- D. Golovaty and S. Talbott, Polygonization of carbon nanotubes, 2013, http://xxx.tau.ac.il/pdf/0711.1719.pdf Search PubMed
.
- D. Golovaty and S. Talbott, Continuum model of polygonization of carbon nanotubes, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 081406 CrossRef
.
- K. Behler, S. Osswald, H. Ye, S. Dimovski and Y. Gogotsi, Effect of thermal treatment on the structure of multi-walled carbon nanotubes, J. Nanopart. Res., 2006, 8, 615–625 CrossRef CAS
.
- O. Hod, Graphite and Hexagonal Boron-Nitride have the Same Interlayer Distance. Why?, J. Chem. Theory Comput., 2012, 8, 1360–1369 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2014 |