Selective iodide chemosensing through a redox-active Cu-corrole†
Abstract
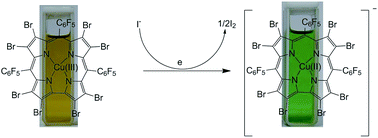
An electron deficient Cu(III)-corrole (B) has been identified as a highly selective colorimetric sensor for iodide. A one-electron redox couple between iodide and Cu(III)-corrole serves as the basis for selectivity. The proposed mechanism has been supported by UV-vis and 19F NMR studies and further by unambiguous identification of Cu(II) species through EPR.

 Please wait while we load your content...
Please wait while we load your content...