Facile synthesis of a reduced graphene oxide/cobalt sulfide hybrid and its electrochemical capacitance performance
Kai Daia,
Dongpei Lia,
Luhua Lu*b,
Qi Liu*c,
Jiali Lva and
Guangping Zhua
aCollege of Physics and Electronic Information, Huaibei Normal University, Huaibei, 235000, P.R. China
bState Key Lab of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan, 430070, P.R. China. E-mail: lhlu@whut.edu.cn
cLaboratory of Nano-Fabrication and Novel Devices Integrated Technology, Institute of Microelectronics, Chinese Academy of Sciences, Beijing, 100029, P.R. China. E-mail: liuqi@ime.ac.cn
First published on 19th June 2014
Abstract
In this work, reduced graphene oxide (RGO) in situ composites with cobalt sulfide (CoS) are achieved through a facile hydrothermal approach. The morphology and structure of the composite materials have been investigated by using scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray photoelectron spectra (XPS) and X-ray diffraction (XRD). The results have shown that the composites consist of CoS nanoparticles with a diameter of 30–50 nm uniformly dispersed on the basal plane of RGO. The maximum specific capacitance of 1130 F g−1 measured by chronopotentiometry at a current density of 0.5 A g−1 is obtained in a 6 M KOH aqueous solution, which is 2.4 times higher than that of pure CoS nanoparticle electrodes (473 F g−1). Furthermore, RGO/CoS nanocomposite exhibits good cycling stability with 92.1% capacitance retention over 1000 cycles.
1. Introduction
With the ever-increasing energy and power consumption in applications ranging from portable electronic tools to vehicle electrification and large industrial equipment, intense research efforts have been devoted to developing the technologies for energy conversion and storage, which require high power uptake or delivery and a long cycle life.1 Of the various power source devices, electrochemical capacitors (ECs), also known as ultracapacitors or supercapacitors, have received great interest for satisfying future renewable energy technologies because of their excellent properties such as cheap, high power, effective, environmentally friendly, long cyclic life and fast charge–discharge rates.2Over the past decade, nanostructured metal sulfides have been extensively investigated as electrode materials for EC design because the good performance provided by redox reactions of electrode usually take place in metal sulfides where metal ions have multiple valence states. Sb2S3,3 In2S3,4 Cu2−xS,5 MoS2,6 CoS,7 Bi2S3,8 KCu7S4,9 and Ni3S2 (ref. 10) nanoparticles are widely used in ECs or rechargeable lithium batteries. Among them, CoS has been studied for its relative low-cost, excellent capacitances, low toxicities and large surface sizes than other materials.11 Nevertheless, CoS nanoparticle does not deliver ideal specific capacitance for its poor electrical conductivity and poor cycling stability.12 Accordingly, improving electrochemical capacitance substantially to meet the requirements of future practical applications and advancing our understanding of the electrochemical interfaces by developing composite materials at the nanoscale are urgently desirable.
Currently, carbonaceous materials, such as carbon nanotubes,13 activated carbon,14 carbon aerogels,15 activated carbon nanofibers,16 and template carbon foams,17 etc., have been frequently chosen as a component for EC materials of effectively improved electrical conductivity and electrochemical stability. Graphene, a two-dimensional nanosheet of graphite, has recently received rapidly growing attention in ECs as it possesses superior electron transport capabilities, a high theoretical surface area (2630 m2 g−1), excellent mechanical flexibility, high thermal and chemical stability, and easy functionalization make graphene good substrate to produce graphene-based functional composites.18 In recent years, many studies covering graphene and related materials used for EC materials have been published. For example, Chidembo et al. reported the capacitances of reduced graphene oxide (RGO) with Co3O4 or NiO nanocomposites are 687 F g−1 and 656 F g−1,19 which are much higher than that of Co3O4 or NiO. Chen et al. obtained hybrid nanostructures of MnO2/graphene nanosheets for high-performance supercapacitor.20 Qu et al. have initially shown that the composite of β-CoS nanoparticles decorated graphene has attractive energy storage performance.21 For the promising energy storage performance that other composites seldom achieved, detailed investigating structure character and understanding performance variation mechanism of metal sulfide and graphene composite structure is important.
In this work, we detailed investigated uniform RGO/CoS nanocomposite formation in aqueous solution via hydrothermal reaction. Electrochemical cyclic voltammeter and galvanostatic charge–discharge measurement in combined with porous structure analysis and EIS analysis have been done to understand the mechanism of electrochemical energy performance variation of RGO/CoS nanocomposite in compared with pure CoS.
2. Experimental
2.1 Materials
Natural graphite powder (325 mesh), acetylene black and polytetrafluoroethene (PTFE) was commercially obtained from Alfa-Aesar. Hydrochloric acid (37%, HCl), concentrated sulfuric acid (98%, H2SO4), potassium hydroxide (KOH) and potassium permanganate (KMnO4) were purchased from Shanghai Chemical Reagent Co., Ltd (P.R. China). Sodium nitrate (NaNO3), ethylene glycol, potassium persulfate (K2S2O8), phosphorus pentoxide (P2O5), hydrogen peroxide (30%, H2O2), thioacetamide and cobaltous nitrate (Co(NO3)2·6H2O) were purchased from Sinopharm Chemical Reagent Corp (P.R. China). All reactants were used as raw materials without further purification. Double distilled water was used during the experimental process. The experiments were carried out at room temperature and humidity.2.2 Preparation of GO
GO was prepared by modified Hummers' method.22 In detail, 3.2 g graphite was put into a mixture of 15 mL H2SO4, 2.5 g K2S2O8, and 2.5 g P2O5. The solution was heated to 80 °C and kept stirring for 5 h in oil bath. Then the mixture was diluted with 700 mL water, and the product was obtained by filtering using 0.2 μm Nylon film and dried under room temperature. Thereafter, 15.0 g KMnO4 was slowly added with magnetic stirring, to prevent the temperature of the mixture from exceeding 10 °C by ice bath. Then ice bath was then removed and the mixture was stirred at 35 °C for 2 h. The reaction was terminated by adding 800 mL of water and 25 mL H2O2 solution. Finally, the GO was obtained by filtration and drying.2.3 Synthesis of RGO/CoS composite
1.2 g Co(NO3)2·6H2O, 0.34 g thioacetamide, 1.0 g PVP, 40 μL HCl and 0.03 g GO were initially dissolved in 20 mL water and 20 mL ethylene glycol and ultrasonicated for 1 h. And then, the mixture was further transferred into a 50 mL Teflon-lined autoclave and subsequently heated at 180 °C for 12 h. When it cooled down to room temperature naturally, the RGO/CoS nanocomposites were harvested by centrifugation and washed with water, and were finally dried at 60 °C for 6 h. The weight ratio of CoS in the composite is 93%.2.4 Characterization
X-ray diffraction (XRD) patterns of CoS and RGO/CoS were carried out using a Rigaku D/MAX 24000 diffractometer with Cu Kα radiation (λ = 1.54056 Å) and a scanning speed of 0.02° s−1 in the 2θ range from 5° to 60°. The accelerating voltage and emission current were 40 kV and 40 mA, respectively. The morphologies of CoS and RGO/CoS were checked using a scanning electron microscopy (SEM, JSM-6700F) at an accelerating voltage of 30 kV equipped with an Inca energy dispersive spectrometer (EDS). The fine structure of the RGO/CoS composite was further investigated on a Tecnai G2 F20 S-Twin transmission electron microscope (TEM) with an accelerating voltage of 200 kV. Core level analysis of the RGO/CoS composite was conducted on an X-ray photoelectron spectrum (XPS) using a Kratos AXIS Ultra DLD X-ray photoelectron spectrometer at room temperature with 1486.6 eV X-ray from the Al Kα line. The Brunauer–Emmett–Teller (BET) specific surface area values were determined by using N2 adsorption data at 77 K obtained by a Micromeritics ASAP 2010 system with multipoint BET method.2.5 Electrochemical measurements
The electrochemical experiments were carried out by a three-electrode system at room temperature on a computer-controlled Shanghai Chenhua CHI 660D workstation. The working electrodes were fabricated by mixing 15 wt% acetylene black, 80 wt% active materials and 5 wt% PTFE binder. Afterward, the mixture was diluted with a small amount of deionized water to form homogeneous mixture slurry. After a short period of drying by evaporation, 5 mg resulting paste was pressed into 10 mm × 10 mm nickel grid under a pressure of 1.2 × 107 Pa for 30 min. The electrode was then dried for 6 h at room temperature. The electrochemical tests were carried out using 6 M KOH aqueous solution as electrolyte. Before the measurements, the CoS-based electrodes were soaked in 6 M KOH aqueous solution for 10 h. A platinum foil and a saturated calomel electrode (SCE) were used as the counter and reference electrodes, respectively. The galvanostatic charge–discharge process was carried out with different current densities. The specific capacitance (C [F g−1]) of the electrode materials were calculated from the discharge curve according to the following equations:23| C = IΔt/(ΔVm) | (1) |
3. Results and discussion
Fig. 1 present the designed formation processes for obtaining RGO/CoS nanocomposites. At the stage (I), the sufficient exfoliation of the graphite into GO nanosheets in water, GO nanosheets have their basal planes decorated mostly with epoxy and hydroxyl groups, while carbonyl and carboxyl groups are located at the edges by the modified Hummers' method. These functional groups enable the subsequent in situ formation of nanostructures attaching on the surfaces and edges of GO nanosheets. At the stage (II), Co2+ ions, formed by the dissolution of Co(NO3)2·6H2O, favorably bind with the O atoms of the negatively charged oxygen-containing functional groups on GO sheets via an electrostatic attraction. At the stage (III), with the help of thioacetamide under condition of 180 °C, large number of CoS nuclei were formed in a short time from the redox reaction occurring between Co2+ and H2S. The chemical reaction involved in the growth of CoS is according to the following equations:
 | (2) |
 | (3) |
 | (4) |
The CoS molecules may form bonds with O atoms of the functional groups via an intermolecular hydrogen bond or a covalent coordination bond, acting as anchor sites for the crystals growth, and the strong chemical and/or physical absorption of CoS nanocrystals on GO nanosheets after the hydrothermal reaction, and at the same time, GO was reduced to RGO.
Fig. 2 shows the XRD patterns of GO, CoS and RGO/CoS composites, respectively. For GO, the XRD peak at 10° for the (001) facets corresponds to 0.87 nm interlayer spacing, which is much larger than that of pristine graphite (0.34 nm) due to the introduction of oxygen-containing functional groups on the graphite sheets.24 The diffraction angles at 2θ = 30.62°, 35.50°, 47.02° and 54.86°, can be assigned to (1 0 0), (1 0 1), (1 0 2) and (1 1 0) crystal planes of pure CoS with the hexagonal structures and the lines match well with the value reported by JCPDS (no. 75-0605, a = 0.338 nm and c = 0.515 nm).
Fig. 3a and b show the typical SEM images of CoS and RGO/CoS composites, respectively. The size of the as-synthesized CoS nanoparticles was ca. 30–50 nm, and the RGO sheets and the nanostructures of CoS particles can be observed, signifying the successful incorporation of CoS nanoparticles onto RGO sheets for a composite. Fig. 3c and d show the EDS spectra of CoS and RGO/CoS composites, respectively. The pattern indicates that CoS crystals only contain elements of Co and S, without any other impurities, and the as-synthesized RGO/CoS composites only contain elements of C, Co, S and O. Fig. 3e and f show the typical TEM images of GO and RGO/CoS composites, respectively. The binding between RGO and CoS surfaces was tight enough to resist repeated rinsing and ultrasonication.
 | ||
| Fig. 3 SEM images of (a) CoS and (b) RGO/CoS composites, EDS spectra of (c) CoS and (d) RGO/CoS composites and TEM images of (e) GO and (f) RGO/CoS composites. | ||
The XPS spectrum including signals for C1s, Co2p, O1s and S2p of RGO/CoS composites to probe the chemical environment of the elements in the near surface range has shown in Fig. 4a. As indicated in Fig. 4b, the asymmetrical and broad features of the observed C1s peaks suggest the co-existence of distinguishable models. Deconvolution core level spectra at about 284.82, 286.88 and 288.72 eV have been giving. The sharp peak located at 284.82 eV is attributed to sp2-hybridized carbons (C–C).25 While the peak at 286.88 eV is ascribed to the existence of C–OH bonds,26 and the relatively weak peak at 288.72 eV is ascribed to the existence of C–OOH bonds.27 Fig. 4c shows the high-resolution spectra of O1s. In the case of RGO/CoS nanocomposite, the curve fitting of O1s spectrum basically indicates two components centered at 529.44 and 531.42 eV, which are commonly ascribed to the surface oxygen complexes of carbon phase.28 Fig. 4d shows the high-resolution spectra of Co2p, the peaks at 794.24 and 779.16 eV correspond to the Co2p1/2 and Co2p3/2 spin–orbit peaks of CoS,29 and a broadened peak at 800.26 eV can be attributed to satellite signal. As indicated in Fig. 4e, the peak centered at 162.1 eV corresponds with the binding energies of Co–S.30
 | ||
| Fig. 4 The overview (a) and the corresponding high-resolution XPS spectra (b) C1s, (c) O1s, (d) Co2p and (e) S2p of RGO/CoS composites. | ||
To enhance the utilization of EC material for achieving high capacitance, it is vital to design material structures with large surface areas for efficient access of electrolyte ions. Fig. 5 shows the N2 adsorption–desorption isotherms for CoS and RGO/CoS nanocomposites at 77 K. The data of BET surface area, pore specific volume of samples are listed in Table 1. The N2 sorption isotherm for the RGO/CoS sample displays hysteresis loops at relative pressures (P/P0) close to unity, indicating the presence of large mesopores and macropores. Table 1 shows that RGO/CoS composite samples have much larger specific surface areas than pure CoS. This is due to the presence of RGO in the composites, which has an extremely high surface area.
 | ||
| Fig. 5 Isotherms for Nitrogen adsorption–desorption of CoS and RGO/CoS composites (Inset: pore size distribution of RGO/CoS). | ||
| Samples | BET surface area (m2 g−1) | Total pore volume (cm3 g−1) |
|---|---|---|
| CoS | 1.4 | 0.009 |
| RGO/CoS | 92.6 | 0.143 |
Fig. 6a and b present the cyclic voltammetry (CV) curves of CoS and RGO/CoS electrodes within the electrochemical window from −0.1 to 0.6 V under the scan rate of different potential scan rates ranging from 1 to 50 mV s−1. The two anodic peaks are likely due to the oxidations of CoS to CoSOH and CoSOH to CoSO, separately:31
 | (5) |
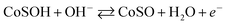 | (6) |
The two oxidized peaks of the as-prepared CoS nanomaterial and RGO/CoS composite occur separately at range of 0.1–0.2 V and 0.4–0.5 V. Scan rate and the shape and the structure of electrode material will influence the redox peaks.29,32 The current CV density of CoS-based materials gradually increased with the increase of scan rate, RGO/CoS electrode yields the largest current and results in much higher capacitance. The reasons can be explained as the follows: (a) the synergistic effects from RGO and CoS nanoparticle of composite electrodes that further boosts the electrical conductivity and contributes the redox-based pseudocapacitance; (b) the charge–discharge process of RGO/CoS composite in the KOH aqueous electrolyte was mainly governed by the insertion of ions from the electrolyte into almost all available area of the electrode and its release to the electrolyte, which can be facilitated by the special 2-dimensional structure.
Galvanostatic charge–discharge measurements were also carried out to assess the potential of RGO/CoS composite as electrode materials for ECs. Fig. 7a and b show the charge–discharge curves for the synthesized CoS and RGO/CoS at varied current densities (from 0.5 A g−1 to 5 A g−1) within a voltage range between 0.0 and 0.5 V, respectively. According to eqn (1), the corresponding specific capacitances of CoS and RGO/CoS composites are shown in Fig. 7c. The theoretical capacity of CoS is about 1300 F g−1,33 but CoS nanoparticles can not deliver ideal specific capacitance because of the poor electrical conductivity. RGO/CoS composite exhibits the maximum specific capacitance of 1130 F g−1 at 0.5 A g−1, which is 2.4 times that of pure CoS nanoparticles (473 F g−1). The coulombic efficiency (CE) were calculated by discharge capacity/charge capacity, CE is 92%, 88%, 74% and 64% for CoS at 0.5 A g−1, 1 A g−1, 3 A g−1 and 5 A g−1, respectively, and 97%, 92%, 87% and 80% for RGO/CoS at 0.5 A g−1, 1 A g−1, 3 A g−1 and 5 A g−1, respectively. Thus, CoS effectively attaches to the surfaces of conductive RGO can effectively make its intrinsic electrochemical capacitance well embodied. Moreover, RGO supplies sufficient electrochemically active sites for effective redox reactions on the CoS surfaces and provided effective channels for the deep intercalation or de-intercalation of ions, which is beneficial to the fast transfer of ions throughout the whole electrode, consequently improving electrochemical performance.34 Thus RGO/CoS composite exhibits attractive high specific capacitance because of the synergistic effect of the pseudocapacitive of CoS and high conductive and large surface area of RGO. To understand the electrochemical performance characteristics of RGO/CoS composites, we performed EIS measurements on these samples as well as CoS nanoparticles sample. Fig. 7d shows the Nyquist plots obtained from the EIS measurements. The EIS data are fitted based on an equivalent circuit model that consists of a bulk solution resistance RS, electrode resistance Re, a charge-transfer resistance Rct, a capacitive element Cdl, and a Warburg element (W).35 Accordingly, RS is 0.23 Ω, the Re of the CoS and RGO/CoS is 1.36 and 0.23 Ω, respectively, whereas Rct is 2.12 and 0.22 Ω in the same order. As is well known, the electrochemical capacitor performance is closely related to the charge-transfer resistance Rct. The higher charge-transfer resistance corresponds to the lower specific capacitance. This clearly shows that the RGO/CoS composite displays much more favorable charge-transfer kinetics than CoS. Furthermore, the conducting agent obviously affects the specific capacitance of the EC in the CoS-based electrode.
Fig. 8 shows the electrochemical stability of the RGO/CoS and CoS electrodes in 6 M KOH electrolyte by galvanostatic charging–discharging at a current density of 0.5 A g−1 within a voltage range of 0.0 and 0.5 V for 1000 cycles. 92.1% of the initial capacitance of RGO/CoS is preserved after 1000 charge–discharge cycles, but CoS nanoparticles only retains 78.2% of initial capacitance after 1000 cycles. The unique structure of RGO/CoS nanocomposite effectively prevents the aggregation of RGO and CoS and consequently provides high specific surface area, which is favorable for fast hydrated ion transport in the electrolyte to the surfaces of both RGO and CoS nanoparticles. Furthermore, the superior electrical conductivity of RGO can significantly decrease the internal resistance of electrode by the construction of a conductive network. CoS nanoparticles with small diameters are also helpful for shortening ion diffusion path, which can greatly reduce the charge transfer resistance and ionic diffusion resistance.36 This kind of relatively low-cost RGO/CoS nanocomposite combines high capacitance and long cycling life. Therefore, the present RGO/CoS nanocomposite could be designed to meet the demand for EC electrodes.
4. Conclusions
A promising route for easy and large scale synthesis of RGO/CoS nanocomposite is developed. The CoS nanoparticles with a size of 30–50 nm are uniformly anchored on both sides of the RGO nanosheets. The as-synthesized RGO/CoS nanocomposite exhibits attractive specific capacitance as high as 1130 F g−1 at 0.5 A g−1 and 92.1% maintenance effect after 1000 cycles of electrochemical charge–discharge. The improvement in cycling stability is attributed to the introduction of the flexible graphene that not only buffers the volume changes and but also prevents CoS nanoparticles from aggregating. The introduced graphene also offers 2D conductive networks and enhances the charge transfer rate, leading to enhanced rate capability.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51302101, 21303129), the Natural Science Foundation of Anhui Province (1408085QE78), the key Foundation of Educational Commission of Anhui Province (KJ2012A250), and the Huaibei Science and Technology Development Funds (20110305).Notes and references
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed; P. Simon and Y. Gogotsp, Nat. Mater., 2008, 7, 845 CrossRef PubMed; X. Yang, C. Cheng, Y. Wang, L. Qiu and D. Li, Science, 2013, 341, 534 CrossRef PubMed; Q. Lu, J. G. Chen and J. Q. Xiao, Angew. Chem., Int. Ed., 2013, 52, 1882 CrossRef PubMed; A. S. Aricò, P. Bruce, B. Scrosati, J.-M. Tarascon and W. V. Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef PubMed.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797 RSC; Q. Lu, M. W. Lattanzi, Y. Chen, X. Kou, W. Li, X. Fan, K. M. Unruh, J. G. Chen and J. Q. Xiao, Angew. Chem., Int. Ed., 2011, 123, 6979 CrossRef CAS; L. Zhang, F. Zhang, X. Yang, G. Long, Y. Wu, T. Zhang, K. Leng, Y. Huang, Y. Ma, A. Yu and Y. Chen, Sci. Rep., 2013, 3, 1408 Search PubMed; L. Bao, J. Zang and X. Li, Nano Lett., 2011, 11, 1215 CrossRef PubMed; M. Wu, J. Gao, S. Zhang and A. Chen, J. Power Sources, 2006, 159, 365 CrossRef PubMed.
- B. N. Reddy, M. Deepa and A. G. Joshi, Phys. Chem. Chem. Phys., 2014, 16, 2062 RSC.
- S. Acharya, S. Sarkar and N. Pradhan, J. Phys. Chem. Lett., 2012, 3, 3812 CrossRef CAS.
- N. J. Freymeyer, P. D. Cunningham, E. C. Jones, B. J. Golden, A. M. Wiltrout and K. E. Plass, Cryst. Growth Des., 2013, 13, 4059 CAS.
- K.-J. Huang, L. Wang, Y.-J. Liu, Y.-M. Liu, H.-B. Wang, T. Gan and L.-L. Wang, Int. J. Hydrogen Energy, 2013, 38, 14027 CrossRef CAS PubMed; G. Ma, H. Peng, J. Mu, H. Huang, X. Zhou and Z. Lei, J. Power Sources, 2013, 229, 72 CrossRef PubMed.
- H. Wan, X. Ji, J. Jiang, J. Yu, L. Miao, L. Zhang, S. Bie, H. Chen and Y. Ruan, J. Power Sources, 2013, 243, 396 CrossRef CAS PubMed.
- Z. Zhang, C. Zhou, L. Huang, X. Wang, Y. Qu, Y. Lai and J. Li, Electrochim. Acta, 2013, 114, 88 CrossRef CAS PubMed.
- S. Dai, Y. Xi, C. Hu, J. Liu, K. Zhang, X. Yue and L. Cheng, J. Mater. Chem. A, 2013, 1, 15530 CAS.
- C.-S. Dai, P.-Y. Chien, J.-Y. Lin, S.-W. Chou, W.-K. Wu, P.-H. Li, K.-Y. Wu and T.-W. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 12168 CAS.
- F. Tao, Y.-Q. Zhao, G.-Q. Zhang and H.-L. Li, Electrochem. Commun., 2007, 9, 1282 CrossRef CAS PubMed; G. Huang, T. Chen, Z. Wang, K. Chang and W. Chen, J. Power Sources, 2013, 235, 122 CrossRef PubMed; M.-R. Gao, Y.-F. Xu, J. Jiang and S.-H. Yu, Chem. Soc. Rev., 2013, 42, 2986 RSC; J. Pu, Z. Wang, K. Wu, N. Yu and E. Sheng, Phys. Chem. Chem. Phys., 2014, 16, 785 RSC.
- J.-Y. Lin, Y.-T. Tsai, S.-Y. Tai, Y.-T. Lin, C.-C. Wan, Y.-L. Tung and Y.-S. Wu, J. Electrochem. Soc., 2013, 160, D46 CrossRef CAS PubMed.
- K. Dai, C. Liang, L. Lu, J. Dai, G. Zhu, Z. Liu, Q. Liu and Y. Zhang, Mater. Chem. Phys., 2014, 143, 1344 CrossRef CAS PubMed.
- X. Du, C. Wang, M. Chen, Y. Jiao and J. Wang, J. Phys. Chem. C, 2009, 113, 2643 CAS.
- A. Halama, B. Szubzda and G. Pasciak, Electrochim. Acta, 2010, 55, 7501 CrossRef CAS PubMed.
- V. Barranco, M. A. Lillo-Rodenas, A. Linares-Solano, A. Oya, F. Pico, J. Ibañez, F. Agullo-Rueda, J. M. Amarilla and J. M. Rojo, J. Phys. Chem. C, 2010, 114, 10302 CAS.
- Y. Lv, L. Gan, M. Liu, W. Xiong, Z. Xu, D. Zhu and D. S. Wright, J. Power Sources, 2012, 209, 152 CrossRef CAS PubMed.
- D. Chen, L. Tang and J. Li, Chem. Soc. Rev., 2010, 39, 3157 RSC; D. Li, M. B. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101 CrossRef CAS PubMed; Y. Zhao, J. Liu, Y. Hu, H. Cheng, C. Hu, C. Jiang, L. Jiang, A. Cao and L. Qu, Adv. Mater., 2013, 25, 591 CrossRef PubMed; X. Zhao, C. M. Hayner, M. C. Kung and H. H. Kung, Adv. Energy Mater., 2011, 1, 1079 CrossRef; K. Dai, L. Lu, C. Liang, J. Dai, Q. Liu, Y. Zhang, G. Zhu and Z. Liu, Electrochim. Acta, 2014, 116, 111 CrossRef PubMed.
- A. Chidembo, S. H. Aboutalebi, K. Konstantinov, M. Salari, B. Winton, S. A. Yamini, I. P. Nevirkovets and H. K. Liu, Energy Environ. Sci., 2012, 5, 5236 CAS.
- S. Chen, J. Zhu, X. Wu, Q. Han and X. Wang, ACS Nano, 2010, 4, 2822 CrossRef CAS PubMed.
- B. Qu, Y. Chen, M. Zhang, L. Hu, D. Lei, B. Lu, Q. Li, Y. Wang, L. Chen and T. Wang, Nanoscale, 2012, 4, 7810 RSC.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS; Y. Wang, Y. M. Li, L. H. Tang, J. Lu and J. H. Li, Electrochem. Commun., 2009, 11, 889 CrossRef PubMed.
- C. Guan, X. Li, Z. Wang, X. Cao, C. Soci, H. Zhang and H. J. Fan, Adv. Mater., 2012, 24, 4186 CrossRef CAS PubMed; B. Gao, C. Yuan, L. Su, L. Chen and X. Zhang, J. Solid State Electrochem., 2008, 13, 1251 CrossRef.
- Q. Liu, J. Shi, J. Sun, T. Wang, L. Zeng and G. Jiang, Angew. Chem., Int. Ed., 2011, 123, 6035 CrossRef.
- R. Bhowmick, S. Rajasekaran, D. Friebel, C. Beasley, L. Jiao, H. Ogasawara, H. Dai, B. Clemens and A. Nilsson, J. Am. Chem. Soc., 2011, 133, 5580 CrossRef CAS PubMed.
- H. Ago, T. Kugler, F. Cacialli, W. R. Salaneck, M. S. P. Shaffer, A. H. Windle and R. H. Friend, J. Phys. Chem. B, 1999, 103, 8116 CrossRef CAS.
- K. Dai, G. Dawson, S. Yang, Z. Chen and L. Lu, Chem. Eng. J., 2012, 191, 571 CrossRef CAS PubMed.
- K. Dai, L. Lu, Q. Liu, G. Zhu, Q. Liu and Z. Liu, Dalton Trans., 2014, 43, 2202 RSC.
- C.-Y. Chen, Z.-Y. Shih, Z. Yang and H.-T. Chang, J. Power Sources, 2012, 215, 43 CrossRef CAS PubMed.
- S.-J. Bao, Y. Li, C. M. Li, Q. Bao, Q. Lu and J. Guo, Cryst. Growth Des., 2008, 8, 3745 CAS.
- Z. Yang, C.-Y. Chen and H.-T. Chang, J. Power Sources, 2011, 196, 7874 CrossRef CAS PubMed.
- S.-J. Bao, C. M. Li, C.-X. Guo and Y. Qiao, J. Power Sources, 2008, 180, 676 CrossRef CAS PubMed.
- R. D. Apostolova, E. M. Shembel, I. Talyosef, J. Grinblat, B. Markovsky and D. Aurbach, Russ. J. Electrochem., 2009, 45, 311 CrossRef CAS; A. Débart, L. Dupont, R. Patrice and J. M. Tarascon, Solid State Sci., 2006, 8, 640 CrossRef PubMed; W. Luo, Y. Xie, C. Wu and F. Zheng, Nanotechnology, 2008, 19, 075602 CrossRef PubMed; J. M. Yan, H. Z. Huang, J. Zhang, Z. J. Liu and Y. Yang, J. Power Sources, 2005, 146, 264 CrossRef PubMed.
- Y. J. Mai, X. L. Wang, J. Y. Xiang, Y. Q. Qiao, D. Zhang, C. D. Gu and J. P. Tu, Electrochim. Acta, 2011, 56, 2306 CrossRef CAS PubMed; M. Zhang, D. Lei, Z. Du, X. Yin, L. Chen, Q. Li, Y. Wang and T. Wang, J. Mater. Chem., 2011, 21, 1673 RSC.
- H.-Y. Wu and H.-W. Wang, Int. J. Electrochem. Sci., 2012, 7, 4405 Search PubMed; T. Y. Wei, C. H. Chen, K. H. Chang, S. Y. Lu and C. C. Hu, Chem. Mater., 2009, 21, 3228 CrossRef CAS.
- C. Jiang, E. Hosono and H. Zhou, Nano Today, 2006, 1, 28 CrossRef.
| This journal is © The Royal Society of Chemistry 2014 |





