Nitroalkenes in the synthesis of carbocyclic compounds
Azim Ziyaei Halimehjani
*a,
Irishi N. N. Namboothiri
*b and
Seyyed Emad Hooshmand
a
aFaculty of Chemistry, Kharazmi University, 49 Mofateh St., Tehran, Iran. E-mail: ziyaei@khu.ac.ir; Fax: +98 (21)88820992; Tel: +98 (21)88848949
bDepartment of Chemistry, Indian Institute of Technology Bombay, Mumbai 400 076, India. E-mail: irishi@chem.iitb.ac.in
First published on 24th June 2014
Abstract
The applications of nitroalkenes in the synthesis of small, common and medium ring carbocycles, including natural products are investigated in this review. These carbocyclic compounds were synthesized from cyclic or acyclic nitroalkenes via a wide variety of reactions such as Michael addition, Diels–Alder reaction, 1,3-dipolar and cycloaddition, Morita–Baylis–Hillman reaction and many cascade reactions often with high regio- and stereoselectivities. Nitroalkenes with a variety of substitution patterns including electroneutral, electron donating and electron withdrawing groups at α- and/or β-positions are suitable substrates for the synthesis of the carbocyclic compounds. The high reactivity of nitroalkenes and their ability to coordinate the metal catalysts as well as organocatalysts signify them as efficient substrates in synthetic organic chemistry.
1. Introduction
Olefins activated by a powerful electron withdrawing group such as the nitro group are versatile substrates and intermediates in a variety of transformations in organic synthesis.1 In recent decades, novel methods based on conjugated nitroalkenes as the key substrates have emerged and numerous challenging targets have been achieved.2–7 This was possible primarily due to the ease of preparation or ready availability and the diverse reactivity of nitroalkenes. The commonly employed method for the preparation of nitroalkenes is indeed the nitro-aldol (Henry) reaction.8 However, other methods currently available in the literature, including direct nitration of olefins, nitrodecarboxylation of α,β-unsaturated carboxylic acids, nitration of vinyl boronic acids, cross-coupling reactions etc., have enhanced the accessibility and the potential of nitroalkenes as the substrates of choice in myriad reactions.9From a status of being unpredictable in reactivity due to their aggressive encounters with metal based reagents and propensity to undergo polymerization, nitroalkenes have now carved out a niche in their own right to become one of the most fascinating classes of substrates in synthetic organic chemistry.
The many facets of nitroalkenes are manifested in their ability to react as Michael acceptors,2 dienophiles, dipolarophiles and heterodienes.3 In particular, nitroalkenes have become bench-mark substrates in catalytic asymmetric Michael additions.2 In recent years, nitroalkenes have also been employed extensively as substrates in the Morita–Baylis–Hillman reaction.4 The nitro group per se can be easily transformed to oximes, hydroxylamines, amines, aldehydes and carboxylic acids and is also amenable for substitution and elimination.1 A nitroalkyl moiety is an excellent precursor to reactive 1,3-dipoles such as nitrile oxides and silyl nitronates.10
Although numerous general and focus reviews have appeared in the literature on various aspects of nitroalkene chemistry,1–7 to our knowledge, a review covering the applications of nitroalkenes as substrates in the synthesis of carbocycles is not available in the literature. Therefore, we present here, for the first time, a critical survey of the synthetic potential of nitroalkenes in the construction of small (3–4), common (5–6) and medium (7) ring carbocycles.
2. Synthesis of three-membered carbocycles
Cyclopropane rings are found in the structure of numerous naturally occurring compounds including terpenes, pheromones, fatty-acid metabolites and unusual amino acids.11 Naturally occurring and synthetic cyclopropanes bearing simple or complex functionalities are useful compounds with a wide spectrum of biological properties, including enzyme inhibition, and herbicidal, antimicrobial, insecticidal, antifungal, antibiotic, antibacterial, antitumour and antiviral activities.12 One of the most famous procedures for synthesis of cyclopropane derivatives is conjugate addition to an electrophilic alkene to produce an enolate, which then subsequently undergoes an intramolecular ring-closure. Several molecules such as sulfur ylides, arsonium ylides, α-halo ketones and aldehydes, α-halo nitroalkanes and halomalonates were used successfully as nucleophiles for conjugate addition to electrophilic alkenes to provide the corresponding cyclopropanes.13In this context, Yan et al. reported a Michael addition–intramolecular alkylation sequence reaction for the enantioselective synthesis of nitrocyclopropanes 3 from methyl 2-bromomalonate 2 and nitroolefins1 by using 6′-dimethyl quinine Cat-1 as catalyst at a low catalyst loading of 5 mol%, as shown in Scheme 1.14 Indeed, a range of cyclopropanes could be provided as almost single trans-diastereomers from a variety of aryl and heteroaryl nitroalkenes in excellent enantioselectivities of up to >99% ee combined with good yields. Besides cinchona alkaloids, α,α-(2-naphthyl) prolinol silyl ether Cat-2 was applied by Lattanzi and Russo for this transformation.15 They have found that with using higher amount of catalyst loading (30 mol%), although almost complete trans-diastereoselectivity was observed, but much lower enantioselectivities (≤49% ee) were obtained as shown in Scheme 1.
In addition, recently Kim et al. demonstrated that by using the chiral Ni complex Cat-3 as catalyst in the above reaction, nitrocyclopropane derivatives can be obtained in better yields (70–99%) and excellent diasteroselectivities (>99 de) and enantioselectivities (85–99% ee) (Fig. 1).16
Another one-pot protocol for synthesis of functionalized cyclopropanes 4 was developed by Fan et al. via an efficient oxidative cyclopropanation of the Michael adducts of nitroalkenes 1 with activated methylene compounds 5 by using the combination of iodobenzene diacetate and tetrabutylammonium iodide.17 The thiourea organocatalyst Cat-4 was used as efficient catalyst for asymmetric synthesis of highly functionalized nitrocyclopropanes 4 in moderate to good yields with high diastereo- and enantioselectivity of >90% de and up to 94% ee respectively, as shown in Scheme 2.
 | ||
| Scheme 2 Thiourea catalyzed oxidative cyclopropanation of the Michael adduct of nitroalkenes with activated methylene compounds. | ||
Very recently, an interesting procedure for synthesis of spiro-gem-dichlorocyclopropanes bearing a 1-nitroethyl moiety 6 was reported by Sosnovskikh et al.18 Although it is well known that reactions of the cyclic diketones with nitroalkenes bearing a good leaving group, usually afforded tetrahydrocoumarin derivatives, they described that reaction of 1,3-dicarbonyl compounds 7 with (E)-1,1,1-trichloro-3-nitrobut-2-ene 8 in the presence of sodium acetate provided the target cyclopropanes 6 in good yields and high diastereoselectivities. Several 1,3-cyclohexanediones and dimedones were used successfully in this procedure (Scheme 3).
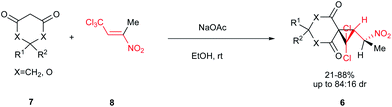 | ||
| Scheme 3 Synthesis of spiro-gem-dichlorocyclopropanes bearing a 1-nitroethyl moiety from (E)-1,1,1-trichloro-3-nitrobut-2-ene. | ||
3. Synthesis of four-membered carbocycles
Substituted cyclobutenes are useful intermediates in organic chemistry19 and are present in the structure of many natural products and biologically active compounds.20Cycloaddition reaction of alkenes and alkynes has become an important synthetic route for construction of highly substituted four-membered carbon ring compounds. In this context, recently, Lam et al.21 reported the first metal catalyzed [2 + 2] cycloadditions of ynamides 9 with nitroalkenes 1, resulting in a range of cyclobutenamide products 10 (Major product) and their diastereomers 11 (minor product) (Scheme 4). The reactions are promoted by substoichiometric quantities of a racemic chiral diene (rac-12)–rhodium complex in conjunction with NaBPh4. The acyclic ynamides 13a-c, in which the nitrogen atom is not part of a cyclic system, were poor substrates in these reactions, generally providing very low conversions.
A tentative catalytic cycle for these reactions is proposed by the authors as depicted in Scheme 5. They assume that the in situ formed unidentified Rh(I) complex 14 can coordinate to the ynamide 9 and nitroalkene 1 to form 15, which then undergoes oxidative cyclization to form a rhodacycle. This rhodacycle can interconvert between the trans isomer 16 and the cis isomer 18 via 17.22 In continuation, reductive elimination of these rhodacycles releases the product 10/11 and regenerates the 14. According to the author opinion, the nature of the active catalytic species 14 and the important role of NaBPh4 in these reactions are not clear.
 | ||
| Scheme 5 Proposed mechanism for [2 + 2] cycloaddition reaction of ynamides with nitroalkenes in the presence of Rh-compex. | ||
Sosnovskikh et al. investigated the reaction of α-(trihaloethylidene)nitroalkanes with enamines.23 They have shown that while the reaction of enamines 19 with CF3-nitroalkenes 20 afforded the [2 + 2] adducts 21 in diethyl ether at −10 °C, the CCl3-nitroalkenes gave no cycloadducts at all and the Michael adducts were obtained in high yields after workup via participation of the methyl group. It is notable that among the diastereoisomers that are possible for cyclobutanes with four contiguous stereogenic centers, the compounds 21 were obtained as single diastereomer in good to high yields with the stereochemistry as shown in Scheme 6. In addition, they have confirmed that while the [2 + 2] products are stable in crystalline state at low temperature for a long time, but in benzene or chloroform at room temperature, they exists as an equilibrium with [4 + 2] cycloaddition adducts 22 and slightly with irreversible formation of the Michael addition adducts 23. Exceptionally, reaction of β-morpholinocrotonates with 20 afforded the cyclic nitronate 22 as the sole product at −10 °C which are also in equilibrium with the [2 + 2]-cycloaddition adducts 21 and the Michael products 23 in solution at room temperature.
Recently, Vicario and coworkers reported an efficient protocol for asymmetric synthesis of substituted cyclobutanes 24 from enolizable α,β-unsaturated aldehydes 25 and α-hydroxymethylnitrostyrenes 26 via [2 + 2] cycloaddition reaction promoted by the combination of Cat-5 (20 mol%) and Schreiner thiourea catalyst Cat-6 (20 mol%).24 The [2 + 2] reaction took place with complete diastereocontrol to give solely a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of α and β anomers 24 (Scheme 7). While high to excellent yield and ee was obtained with β-aryl(heteroaryl)-α,β-unsaturated aldehydes, β-alkyl-substituted α,β-unsaturated aldehyde such as 2-hexenal provided the desired cyclobutane adduct with a somewhat lower yield, but still with same levels of ee. In addition, the cyclobutane adducts were transformed to their corresponding lactones 27 in excellent yields and as single diastereoisomers via oxidation with PCC. The lactone (R, Ar = Ph) was further converted to the corresponding amine 28 via reduction with Zn/AcOH, and tetrasubstituted cyclobutane 29 by base-promoted methanolysis.
1 mixture of α and β anomers 24 (Scheme 7). While high to excellent yield and ee was obtained with β-aryl(heteroaryl)-α,β-unsaturated aldehydes, β-alkyl-substituted α,β-unsaturated aldehyde such as 2-hexenal provided the desired cyclobutane adduct with a somewhat lower yield, but still with same levels of ee. In addition, the cyclobutane adducts were transformed to their corresponding lactones 27 in excellent yields and as single diastereoisomers via oxidation with PCC. The lactone (R, Ar = Ph) was further converted to the corresponding amine 28 via reduction with Zn/AcOH, and tetrasubstituted cyclobutane 29 by base-promoted methanolysis.
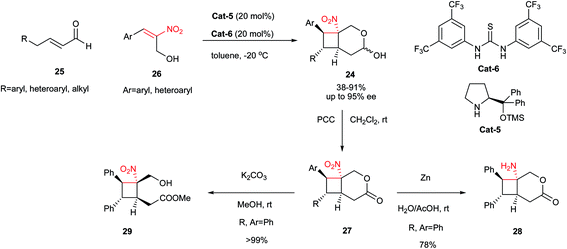 | ||
| Scheme 7 Cascade [2 + 2] cycloaddition/hemiacetalisation reaction for synthesis of four-membered rings. | ||
4. Synthesis of five-membered carbocycles
The development of efficient methods to access functionalized cyclopentanoids is a challenging problem since these valuable building blocks have found wide applications in synthetic organic chemistry and are present as an element in the structure of natural products.25 Traditionally, [3 + 2] annulations are the most efficient protocols for the synthesis of five-membered-ring derivatives.26 Several new routes were also developed for construction of cyclopentanoids which consist of MacMillan procedure, phosphine-catalyzed [3 + 2] cycloadditions of allenoates with electron-deficient olefins, 1,3-dipolar cycloadditions, and electrocyclization.27In recent years, domino, cascade, tandem and sequential reactions have been the focus of current organic chemistry research because they can assemble complex molecule structures from relatively simple starting materials in a one-step process.28 The applications of these reactions in asymmetric version using organocatalysts were also interesting, because high stereoselectivities can be achieved in an operationally simple and straightforward manner.29 Among these strategies, Michael–aldol reaction, double Michael reaction and Michael–Henry reaction are the widely applied approaches for the synthesis of cyclopentanoids. In this section, we will discuss different routes for synthesis of cyclopentanoids starting with nitroalkenes.
4.1. Synthesis of cyclopentane derivatives
Hong et al.30 described that reaction of carbethoxymethylenetriphenylphosphorane 30 (a Wittig reagent) and nitroalkenes 1, followed by a reaction with ethyl formylformate 32 and cinnamaldehydes 34 in the presence of Jørgensen–Hayashi catalyst Cat-5 and HOAc as co-catalyst provided a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric ratio of anti- and syn-pentasubstituted cyclopentanecarbaldehydes 35 bearing a quaternary carbon center with excellent enantioselectivities (up to >99% ee) (Scheme 8). The products were prepared via sequential Michael–Wittig–Michael–Michael reactions. An increase in diastereoselectivity of products (up to 3.2
1 diastereomeric ratio of anti- and syn-pentasubstituted cyclopentanecarbaldehydes 35 bearing a quaternary carbon center with excellent enantioselectivities (up to >99% ee) (Scheme 8). The products were prepared via sequential Michael–Wittig–Michael–Michael reactions. An increase in diastereoselectivity of products (up to 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for anti-isomer) was also achieved by using the noncovalent thiourea catalyst Cat-7 or Cat-8 in the first Michael addition reaction. Using proline as catalyst also affords the 1
1 for anti-isomer) was also achieved by using the noncovalent thiourea catalyst Cat-7 or Cat-8 in the first Michael addition reaction. Using proline as catalyst also affords the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of anti-and syn-isomers in low yield without enantioselectivity.
1 mixture of anti-and syn-isomers in low yield without enantioselectivity.
 | ||
| Scheme 8 Sequential Michael–Wittig–Michael–Michael addition reaction for synthesis of pentasubstituted cyclopentanecarbaldehydes. | ||
The 3-spirocyclopentane-2-oxindoles represent an important class of substructures which are skeleton of a number of biologically active alkaloids31 (Fig. 2) and drug candidates.32
In this context, biologically important spirocyclopentaneoxindoles containing the oxime functional group 36 have been synthesized by Shao et al.33 from easily accessible 3-allyl-substituted oxindoles 37 and nitroolefins via an enantioselective intermolecular Michael addition/intramolecular silyl nitronate-olefin cycloaddition/fragmentation reaction. This approach provided the products with three stereocenters including one spiroquaternary stereocenter in good yields (up to 85%) with excellent diastereoselectivity (up to >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) and enantioselectivity (up to >99% ee). The reaction was efficiently catalyzed by 10 mol% of bifunctional thiourea organocatalyst Cat-9 in CHCl3 at −20 °C (Scheme 9).
1 dr) and enantioselectivity (up to >99% ee). The reaction was efficiently catalyzed by 10 mol% of bifunctional thiourea organocatalyst Cat-9 in CHCl3 at −20 °C (Scheme 9).
 | ||
| Scheme 9 Stereoselective synthesis of spirocyclopentaneoxindoles containing the oxime functional group from nitroalkenes. | ||
Also the same group has developed another approach for synthesis of spirocyclopentaneoxindoles 41 with four contiguous stereocenters including one spiroquaternary stereocenter (Scheme 10).34 They have shown that metathesis reaction of 3-allyloxindole 37 with ethyl acrylate 39 furnished bifunctional oxindole 40 in the presence of Zhan catalyst Cat-10 (5 mol%) in dichloromethane, which undergoes asymmetric double-Michael addition sequence with nitroalkenes in the presence of 10 mol% of Cat-9 to generate the adducts 41 in good yields (72–87%) with excellent diastereoselectivity (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 30
1 → 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) and enantioselectivity (93 → 99% ee). The adducts 41 can be simply converted to biologically interesting tetracyclic molecules 42 possessing indoline, pyrrolidin-2-one, fused [5,5] bicyclic lactams and spirocyclic components by reduction with NaBH4/NiCl2·6H2O.
1 dr) and enantioselectivity (93 → 99% ee). The adducts 41 can be simply converted to biologically interesting tetracyclic molecules 42 possessing indoline, pyrrolidin-2-one, fused [5,5] bicyclic lactams and spirocyclic components by reduction with NaBH4/NiCl2·6H2O.
 | ||
| Scheme 10 Synthesis of spirocyclopentaneoxindoles with four contiguous stereocenters including one spiroquaternary stereocenter. | ||
Bonne et al. reported an asymmetric approach for synthesis of highly functionalized cyclopentanes 43 bearing up to three stereogenic centers with very high stereoselectivity.35 They have shown that the cinchona alkaloid Cat-11 efficiently catalyses the domino reaction occurring between 2-allylmalonates 44 and nitroalkenes 1. The sequence began with the Michael addition of the enolate of 2-allylmalonates on to nitroalkenes, generating the corresponding nitroadducts which were converted to the corresponding nitronates 45a upon treatment with TMSCl. The intramolecular cyclization of 45a with double bond of the allyl moiety furnished the corresponding isoxazolidines 45b which were subsequently submitted to a selective fragmentation by treatment with TBAF to afford the corresponding final cyclopentanone oximes 43 bearing three stereogenic centres in good-to-excellent yields and with high diastereo- and enantioselectivities of up to 97% ee, as shown in Scheme 11. In addition, diastereoselective reduction of cyclopentanone oximes with NaBH3CN affords the corresponding hydroxyl amines as single diastereomers with new stereogenic center in good yields.
 | ||
| Scheme 11 Cinchona alkaloid (Cat-11) catalysed domino reaction occurring between 2-allylmalonates 44 and nitroalkenes 1. | ||
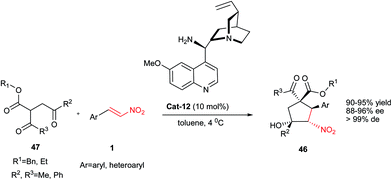
Also, Zhong et al. described another approach for synthesis of highly functionalized cyclopentanes 46 with four stereogenic carbons including two quaternary and two tertiary stereocenters with complete diastereoselectivities and excellent enantioselectivities (88–96% ee) (Scheme 12).36 Under the catalytic influence of Cat-12 in toluene at 4 °C, a carbon nucleophile 47 containing three carbonyl groups reacted with nitroalkene1 to afford adducts 46 in excellent yields (90–95%). This Michael–Henry sequence allows access to chiral enantioenriched cyclopentanes from achiral and easily accessible starting materials.
According to the proposed mechanism by the authors, the two substrates involved in the reaction are activated simultaneously by chiral Cat-12 as shown in the Fig. 3. Nitroolefin assumed to be activated via multiple hydrogen bonding with the primary amine moiety of the catalyst, and the compound 48 assumed to deprotonate with the tertiary amine group of catalyst to generate a highly nucleophilic enolate species which undergoes Michael addition to nitroalkenes. Finally, the subsequent carbanion adjacent to the nitro group attacks the carbonyl group to afford Henry products.
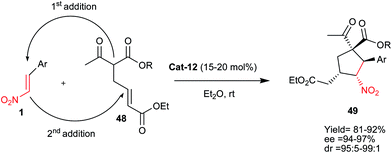
In a parallel study, the same group developed an asymmetric synthesis of polysubstituted cyclopentanes 49 from nitroalkenes 1 and ketoester 48 via double Michael addition reaction (Scheme 13).37 Here, two Michael acceptors are used in the sequence; the nitroalkene 1 and the α,β-unsaturated ester 48. Thus, the reaction is initiated by the addition of the enolate of 1,3-dicarbonyl derivative 48 to the nitroalkene 1 which is slightly more reactive than the α,β-unsaturated ester. The nitronate generated in situ then undergoes cyclization through an intramolecular Michael addition to the α,β-unsaturated ester. The cinchona alkaloid Cat-12 induces very good stereoselectivity for this domino reaction via activation of both the Michael acceptor and donor by hydrogen-bonding interactions.
Very recently, a domino double Michael transformation between alkyl (E)-6-oxohex-2-enoates 50 and nitroalkenes 38 was designed by Liu and Zhang and co-workers (Scheme 14).38 Different reaction conditions were examined and using 10 mol% of Cat-13 in ethanol in the presence of sodium hydrogen carbonate (2.0 equiv.) at 15 °C was selected as optimal condition. It is notable that the Michael adducts were obtained as major products when the reactions were carried out in the presence of an acid additive or additive-free conditions. Under the optimal reaction conditions, the biologically important and synthetically challenging cyclopentanes 51 with four contiguous stereocenters were obtained in good to excellent yields (up to 98%), and excellent diastereoselectivities (up to 100% dr) and enantioselectivities (up to 99% ee). While the aromatic nitroalkenes provided the products in excellent yields, moderate dr's and excellent ee's, aliphatic nitroalkenes afforded the products in lower yield and excellent diastereo- and enantioselectivities.
Bicyclo-[3.2.1]octane derivatives 54 containing four continuous stereogenic centers were prepared by Kokotos et al. via organocatalytic asymmetric reaction between 1,4-cyclohexanedione 52 and nitrodienes 53 (Scheme 15).39 The reaction proceeds through a domino Michael–Henry process to give the corresponding fused cyclopentanes 54 as a single diastereoisomer with excellent enantioselectivities. Among the several bifunctional catalysts, they have shown that only the proline derived catalysts Cat-14 to Cat-16 (10 mol%) are suitable for this transformation. Best yield and selectivity were obtained with Cat-14, and although Cat-16 led to higher yield, the selectivity dropped significantly. Replacing the nitrodienes by aromatic nitroolefins decreases the reaction rate and needs higher amount (20 mol%) of catalyst for completion. Electron-donating or -withdrawing substituents on the phenyl ring of nitroalkenes do not have significant effect on the yield and selectivity of reaction. The presence of 50 μl of water in reaction media proved to be essential for catalyst's turn over.
4.2. Synthesis of methylenecyclopentane derivatives
[3 + 2] Cycloaddition of trimethylenemethanes with olefins is an efficient strategy for synthesis of cyclopentane derivatives. In this context, reaction of 55 with nitroalkenes 38 in the presence of 5 mol% of Pd(dba)2 and chiral phosphoramidite ligand 56 (10 mol%) has been investigated by Trost et al. to give the corresponding nitrocyclopentanes 57 as single diastereomers in high to excellent yield (53–97%) and enantioselectivity (83–95% ee) (Scheme 16).40 Aromatic, heteroaromatic and aliphatic nitroalkenes were found to react equally well in this protocol. Conjugated nitrodiene derived from cinnamaldehyde gave a reaction exclusively at the double bond proximal to the nitro group in moderate yield and high ee. The authors have also shown that these products can be simply transformed to several synthetic intermediates such as cyclopentylamines, cyclopentenones, and cyclopentanes.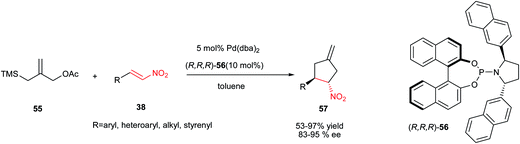 | ||
| Scheme 16 Methylene cyclopentanes via Pd-catalyzed [3 + 2] cycloaddition of trimethylenemethanes with nitroolefins. | ||
Also, the same group described that by using β,β-disubstituted nitroalkenes 58 in this reaction, highly substituted nitrocyclopentanes 60 with a quaternary stereocenter can be obtained in high to excellent yield and enantioselectivity.41 Furthermore, highly substituted cyclopentanes 61 with three contiguous stereocenters can be achieved via reaction of the cyano-substituted TMM donor 59 with β,β-disubstituted nitroalkenes in nearly perfect yield and selectivity (Scheme 17).
Duclere et al. reported a one-pot procedure for diastereoselective synthesis of nitro methylenecyclopentanes 65 and 66 via [3 + 2]-annulation of dimethyl propargylmalonate 64 and nitroalkenes 62 and 63 catalyzed by Triton-B (benzyltrimethylammonium hydroxide) (Scheme 18).42 This strategy does not require activation of the triple bond and involves the direct conversion of a stabilized enolate anion to unstabilized sp2 carbanion. The only by-product found in this procedure is the corresponding Michael adducts, not exceeding 15%. Both cyclic and acyclic nitroalkenes are suitable for this reaction to form the corresponding bicyclic 66 or monocyclic products 65 in moderate to good yields (47–80%) and with excellent levels of stereoselectivity in favor of the cis diastereoisomers. By using t-BuOK as base and t-BuOH as proton donor, the cyclic products were formed in lower yield together with the inseparable Michael adducts. They have also shown that Triton B is the best base for this transformation compared to K2CO3, Cs2CO3, KH, NaH, and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), which proved to be unsuccessful or afforded only poor yields and/or stereoselectivities.
 | ||
| Scheme 18 Triton-B catalyzed diastereoselective synthesis of nitromethylene cyclopentanes via [3 + 2]-annulation of dimethyl propargylmalonate and nitroalkenes. | ||
4.3. Synthesis of cyclopentanol derivatives
Boyce and Johnson43 reported a three-component coupling reaction of a nitroalkene 38, a silyl glyoxylate 67 and a vinylmagnesium bromide 68 to provide functionalized (Z)-silyl enol ethers 69 through a cascade vinylation/[1,2]-Brook rearrangement/vinylogous Michael reaction (Scheme 19). Diastereoselective deprotection/intramolecular Henry sequence of 69 in the presence of NaOH in methanol at −5 °C provided functionalized nitrocyclopentanols 71 with three contiguous stereocenters (see Scheme 19; TES = triethylsilyl). Nitroalkenes substituted with aryl, alkyl, and even a tert-butyl group are suitable starting materials for this transformation.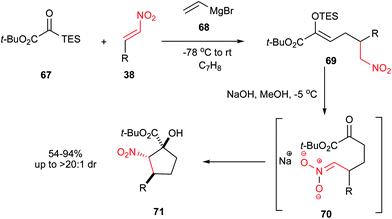 | ||
| Scheme 19 Synthesis of cyclopentanols by vinylation/[1,2]-Brook rearrangement/vinylogous Michael reaction cascade. | ||
4.4. Synthesis of cyclopentanone derivatives
Felluga et al. reported the synthesis of enantiomerically rich 2-hydroxy-3-nitrocyclopentanone derivatives 75 from 2,3-butanedione 72 and conjugated nitroalkenes 62 via [3 + 2] carbocyclization reactions catalysed by (R)-(+) and (S)-(−)-1-phenylethylamine.44 The reaction between chiral amine and dione provide chiral imine 73 which undergoes asymmetric cyclization with nitroalkenes 62 to produce the cyclopentane imine intermediate 74. The imine intermediates 74 were converted into the corresponding α-ketols 75, either by acid hydrolysis under mild conditions or by oxidation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond followed by acidic treatment. The reactions were carried out both in solvent and under solvent-free conditions (Scheme 20).
N bond followed by acidic treatment. The reactions were carried out both in solvent and under solvent-free conditions (Scheme 20).
In addition, bicyclo[3.2.1]otan-8-ones 77 were synthesized in excellent enantioselectivity, high diastereoselectivity, and regioselectivity via a tandem Michael–Henry reaction between cyclohexane-1,2-dione 76 and nitroalkenes 38 (Scheme 21).45 Among the several organocatalysts screened for this reaction, only Quinine-derived thiourea Cat-17 proved to give satisfactory yield and stereoselectivity. Although four stereogenic centers were created during the reaction, only two diastereomers 77a and 77b were obtained in good diastereoselectivity and high enantioselectivity (92–99% ee). On the basis of the X-ray analysis, its stereochemistry or major diastereomers was assigned to be (1R,5R,6S,7S). Cyclopentan-1,2-diones and enolizable acyclic1,2-diones fail to give similar products. Both electron-donating and electron-withdrawing groups on the phenyl ring of nitroalkenes do not lead to significant effect on the dr values, the product yields, or the ee values.
4.5. Synthesis of cyclopentene derivatives
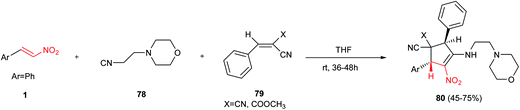
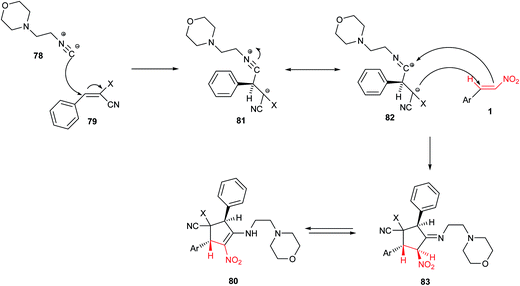
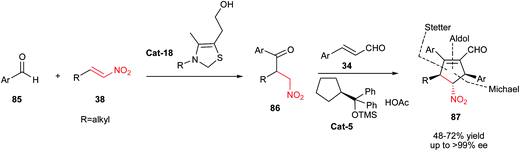
Polysubstituted cyclopentenes 80 were synthesized by Knight et al. via a multicomponent reaction between β-nitrostyrene 1, benzylidenemalononitriles 79 and 2-morpholinoethyl isocyanide 78 (Scheme 22).46 The products were obtained in moderate to good yields under catalyst-free conditions in dry THF. Proposed mechanism by the authors shows that initial attack of isocyanide 78 to benzylidene malononitrile 79 generates an intermediate 81 with 1,4-dipolar structure which is in resonance with 1,3-dipolar structure 82. Dipolar cycloaddition of 82 to β-nitrostyrene affords an imino cyclopentane ring 83, which provides polysubstituted cyclopentenes 80 via further tautomerization (Scheme 23).In another report, the Hong group also described a Stetter–Michael–aldol condensation sequence for synthesis of cyclopentenecarboxaldehydes 87 from aromatic aldehydes 85, nitroalkenes 38, and α,β-unsaturated aldehydes 34 (Scheme 24).47 They have shown that this [1 + 2 + 2] annulation strategy affords the products 87 with excellent diastereoselectivities and enantioselectivities (up to >99% ee). The Stetter reaction was successfully catalyzed by a thiazolium based N-hererocyclic carbene Cat-18 to furnished a β-nitroketone 86 which was used in tandem Michael–aldol condensation with an α,β-unsaturated aldehydes 34 under catalytic amount of acetate salt of Cat-5. Notably, the method delivers substituents to all five locations of the cyclopentene ring with three contiguous chiral centers with excellent stereoselectivity.
In 2010, Shi et al.48 reported another simple and efficient protocol for synthesis of highly functionalized cyclopentenes 89 via a tandem reaction between ethyl 2,3-butadienoate 88 and nitroalkenes 38 catalyzed by P(p-FC6H4)3 (Scheme 25). This process involves a [3 + 2] cycloaddition and a subsequent umpolung addition. The asymmetric version of this tandem reaction was also investigated using (R)-2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (Binap) Cat-19 as the catalyst, which generated the cis-89 in 41% yield and 26% ee. Stronger nucleophilic phosphines such as methyldiphenylphosphine (PPh2Me), dimethyl(phenyl)phosphine (PPhMe2), and tributylphosphine (PBu3) could not catalyze this reaction. Various nitroalkenes with electron-withdrawing or electron-donating groups substituted at ortho-, meta-or para-position of the benzene ring were examined as suitable starting materials for this reaction to afford moderate to good yields of products along with moderate to good diastereoselectivities. Only strongly electron withdrawing group such as the NO2 on the benzene ring gives low yield of product.
Plausible mechanism for this reaction is given in Scheme 26. Reaction of the Lewis base P(p-FC6H4)3 with ethyl 2,3-butadienoate generate the zwitterionic intermediate A, which subsequently undergoes cycloaddition with nitroolefin 38 to afford the intermediate B. Then, a proton transfer from B affords intermediate C which undergo P(p-FC6H4)3 elimination to afford intermediate product D and regenerates P(p-FC6H4)3. Meanwhile, intermediate product D deprotonated by intermediate A to generate intermediates E and F. Subsequent umpolung addition produce intermediate G. G will undergo a hydrogen shift to form intermediate H. Then, the elimination of P(p-FC6H4)3 furnishes product 89 and regenerates the catalyst.
 | ||
| Scheme 26 Proposed mechanism for phosphine-catalyzed tandem reaction of allenoates with nitroalkenes. | ||
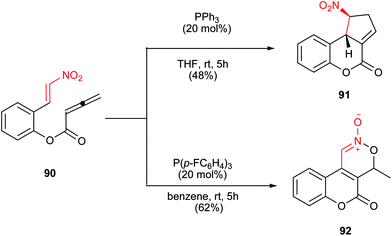
Also the intramolecular version of this reaction is reported by Kwon et al. in 2007 (Scheme 27).49 They have shown that nitrostyrenyl derivative 90 undergoes the intramolecular cyclization in the presence of catalytic amount of PPh3 in THF to produce the fused cyclopentene ring 91 (48%) and 92 (12%). Interestingly, when benzene was used as reaction solvent, the nitronate 92 was obtained as the major product in 58% yield along with a 14% yield of 91. When the reaction was carried out in the presence of less-nucleophilic tris(p-fluorophenyl)phosphine, 92 was obtained in a slightly improved yield (62%).
A one-pot procedure for synthesis of 2-nitro-3-aryl-3H-inden-1-ylamines 96 is reported by Clarke and Hunt starting with β-nitrostyrenes 94 and o-lithiobenzonitrile via a Michael addition reaction at −100 °C, followed by intramolecular nitronate capture by cyano group upon warming up, and tautomerization (Scheme 28).50 The o-lithiobenzonitrile is generated in situ from the corresponding ortho-bromobenzonitrile 93 at −100 °C by bromine–lithium exchange with n-butyllithium in THF. The products were obtained in moderate to good yields. In the case of ortho-methoxy substituent in the nitroalkene, the Michael adduct 97 was also observed as minor by-product which may be because of the stability of the nitronate intermediate upon chelation with the methoxy group.
 | ||
| Scheme 28 A one-pot procedure for synthesis of 2-nitro-3-aryl-3H-inden-1-ylamines from nitroalkenes. | ||
4.6. Synthesis of cyclopentenol derivatives
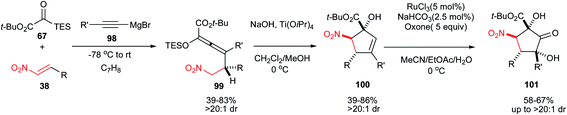
Johnson and coworkers described a one-pot three-component procedure for synthesis of tetrasubstituted silyloxyallene 99 from Mg acetylides 98, silyl glyoxylates 67, and nitroalkenes 38.51 These silyloxyallenes were converted to highly substituted cyclopentenols 100 via a Lewis acid [Ti(O-iPr)4] promoted Henry cyclization in dichloromethane/methanol at 0 °C. Cyclopentenol products 100 were prepared with greater than 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselection. They also have shown that ketohydroxylation of cyclopentenols with oxone in the presence of catalytic amount of RuCl3 gave fully substituted cyclopentanones 101 in high yields (Scheme 29). Aliphatic and aromatic nitroalkenes gave similar results in this transformation.
1 diastereoselection. They also have shown that ketohydroxylation of cyclopentenols with oxone in the presence of catalytic amount of RuCl3 gave fully substituted cyclopentanones 101 in high yields (Scheme 29). Aliphatic and aromatic nitroalkenes gave similar results in this transformation.
4.7. Synthesis of cyclopentenone derivatives
Namboothiri et al. reported that vinylogous Morita–Baylis–Hillman adducts 103 (prepared by reaction of nitroalkenes 1 with methyl vinyl ketone 102 in the presence of imidazole) are suitable starting materials for synthesis of 2,3-disubstituted cyclopentenones 104 (Scheme 30).52 The reaction proceeded via in situ reduction of nitro group by Fe/HCl, hydrolysis of the resulting enamine to ketone and subsequent intramolecular aldol condensation to give the corresponding products in high yields.5. Synthesis of six-membered carbocycles
The cyclohexane ring without any doubt is the most common ring in the nature as a structural fragment of vitamins, hormones, terpenes, alkaloids, and other practically important substances.53 The interest in the synthesis of highly functionalized cyclohexane derivatives is due to the importance of their functions and the possibility of their use as key compounds in the synthesis of biologically important compounds.54Nitroalkenes have been used as efficient starting materials for synthesis of highly functionalized six-membered carbocyclic rings. The most applied routes in these cases are Diels–Alder cycloaddition reaction, cascade Michael–Michael reaction, tandem Michael–Henry reaction, sequential Michael–Michael–Wittig reaction and Michael–Michael–aldol sequence. Also recently several asymmetric catalysts were developed for synthesis of functionalized cyclohexanes with excellent diastereo- and enantioselectivity. Therefore, in this section, we will review the application of nitroalkenes as powerful starting materials for synthesis of functionalized cyclohexanes, cyclohexenes, cyclohexadienes, cyclohexanols, cyclohexanones and other fused cyclohexane derivatives.
5.1. Synthesis of cyclohexane derivatives
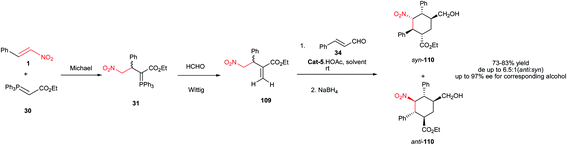
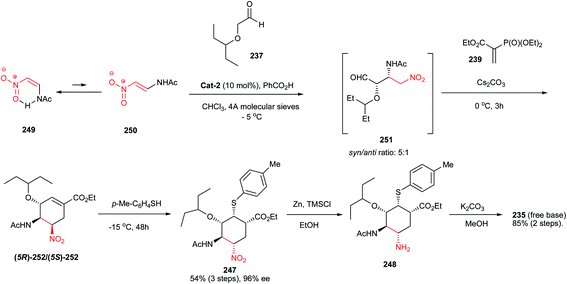
In 2003, Yasuhara et al. showed that addition of aryl lithium 106 to the nitroalkene 105 provide the intermediate nitronate anion which did not add to the enoate group in a tandem process, so that compound 107 is obtained in high yield (Scheme 31).55 This compound then can be cyclized using CsF in THF to afford functionalized cyclohexane 108a/b in poor diastereoselectivity at C-3 but in a complete trans relationship between C-1 and C-2.The same strategy as described in Scheme 8 was also used for synthesis of pentasubstituted cyclohexanes 110 having five contiguous stereocenters with excellent enantioselectivities (Scheme 32).30 Compared to reactions in Scheme 8, they have used formaldehyde instead of ethyl formylformate. The authors mentioned that the originally formed cyclohexanecarbaldehydes are unstable during the isolation and purification process; therefore, the reaction adducts were immediately reduced by NaBH4 to the corresponding alcohols syn-110 and anti-110, and the ee values (up to >97% ee) were determined on the major isomeric alcohol (anti-110). The process shows higher diastereoselectivity toward synthesis of cyclohexane rings compared to cyclopentane rings.
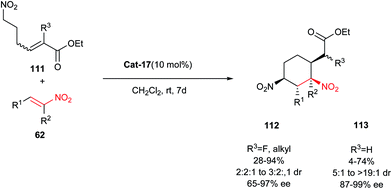
Very recently, an asymmetric domino Michael–Michael reaction between nitrohexenoates 111 and nitroalkenes 62 is reported by Cobb and co-workers for synthesis of optically active functionalized cyclohexanes 112 and 113 bearing up to five contiguous stereocenters (Scheme 33).56 The reaction promoted well with bifunctional thiourea–tertiary amine Cat-17 to give the desired carbocycles with moderate to excellent yields and stereoselectivities. The methodology could be applied to a wide range of aromatic nitroolefins, including trisubstituted nitroalkenes, which formed the expected cycloadduct displaying a quaternary carbon atom in very high stereoselectivities (dr > 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, ee > 99%), despite a very low yield (4%). In addition, trisubstituted nitroenoates (111, R3 = alkyl, F) were also examined and resulted in the formation of cyclohexanes 112 with an additional stereogenic center, although lower yields and diastereoselectivities were observed. Interestingly, the presence of two nitro groups in the structure of products allows further elaborations for synthesis of biologically active compounds.
1, ee > 99%), despite a very low yield (4%). In addition, trisubstituted nitroenoates (111, R3 = alkyl, F) were also examined and resulted in the formation of cyclohexanes 112 with an additional stereogenic center, although lower yields and diastereoselectivities were observed. Interestingly, the presence of two nitro groups in the structure of products allows further elaborations for synthesis of biologically active compounds.
5.2. Synthesis of cyclohexanol derivatives
Highly functionalized cyclohexanol derivatives are suitable intermediates that can be readily transformed into cyclohexenes or cyclohexanones. In this context, Zhong et al. reported the organocatalytic asymmetric tandem Michael–Henry reaction of α-substituted β-ketoesters 114 with nitroolefins 1 to afford the highly functionalized chiral cyclohexanols 115.57 They have shown that 9-amino-9-deoxyepiquinine Cat-12 efficiently catalyze the reaction to give the corresponding products with four stereogenic centers containing two quaternary stereocenters in good to excellent yields (85–94%), excellent enantioselectivities (97% to >99% ee) and high diastereoselectivities (93:7–99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Also they have shown that the position and the electronic property of the substituents on aromatic ring of nitroalkenes do not have significant effect on the stereoselectivities. In addition, the same Michael–Henry adduct 117 was obtained using nitrodiene 53 with 95% yield and 97% ee (Scheme 34).
1 dr). Also they have shown that the position and the electronic property of the substituents on aromatic ring of nitroalkenes do not have significant effect on the stereoselectivities. In addition, the same Michael–Henry adduct 117 was obtained using nitrodiene 53 with 95% yield and 97% ee (Scheme 34).
Another stereoselective procedure for synthesis of substituted nitrocyclohexanols 119 with four stereogenic centers is developed by Hayashi et al. using nitroalkenes 38 and 2,5-dihydroxy-3,4-dihydrofuran as a surrogate for pentane-1,5-dial 118 under 10 mol% of Cat-5 in THF at room temperature (Scheme 35).58 The products 119 were obtained as major distereomers in high yields and excellent enatioselectivity (97–99% ee). The reaction is faster for electron-deficient aryl-substituted nitroalkenes compare to electron-rich aryl-substituted ones. The major product 119 can be simply isomerized to 120 and 121 via treatment with silica gel and DBU respectively, without compromising on the enantioselectivity. Theoretical mechanistic studies and Gibbs free energy calculations were recently carried out by Wong to explain the high stereoselectivity of this Michael–Henry domino reaction.59
A recyclable catalytic system to promote the same Michael–Henry transformation was developed in 2011 by Ni and co-workers with similar results.60 The authors showed that the catalyst could be reused four times without any significant erosion of the rates, yields or stereoselectivities.
Very recently, Hong et al. demonstrated an asymmetric protocol for synthesis of bicyclo[3.3.1]nonane systems 123 from the reaction of 3-aryl-2-nitroprop-2-en-1-ols 122 and glutaraldehyde 118 (25% in water) promoted by 20 mol% of Cat-5 engaged with acetic acid as co-catalyst.61 The enantioenriched 3-oxabicyclo[3.3.1]nonan-2-ones 123 were obtained in good yields and excellent diastereo- and enantioselectivities (Scheme 36). Aromatic nitroalkenes 122 with different electron-donating and -withdrawing groups on the phenyl ring were applied successfully in this protocol.
 | ||
| Scheme 36 Enantioselective synthesis of bicyclo[3.3.1]nonane derivatives through domino Michael–Michael–acetalization reaction. | ||
Interestingly, Hong et al. extended the previous work by replacing the 122 with 2-hydroxy-β-nitrostyrene 124 under similar conditions to allow the generation of an additional carbon–oxygen bond in an efficient Michael–acetalization–Henry cascade (Scheme 37).62 After subsequent oxidation, this sequence gave tetrahydro-6H-benzo[c]chromen-6-ones 125 with high enantiomeric excess values (up to >99% ee) and moderate yields and diastereoselectivities. These products constitute the skeleton of a large range of natural and bioactive molecules.
In 2013, Shi et al. demonstrated the synthesis of nitrocyclohexanols 127 via a cascade Michael–Henry reaction of a β-nitroolefin 1 and a 2-(1-substituted 3-oxo-3-phenylpropyl)malononitrile 126 catalysed by 2′-(diphenylphosphino)biphenyl-2-ol (Cat-20).63 It is notable that compounds 126 can be simply prepared from malononitrile and α,β-unsaturated ketones under 10 mol% of PPh3 in anhydrous CH2Cl2. The most advantages of this work are mild reaction conditions, simple workup, excellent diastereoselectivity (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to >99
5 to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), good atom economy, and a wide substrate scope (Scheme 38).
1), good atom economy, and a wide substrate scope (Scheme 38).
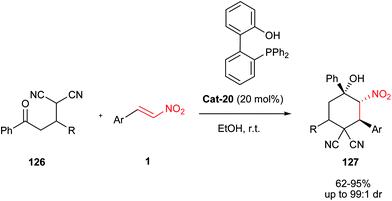 | ||
| Scheme 38 Synthesis of nitrocyclohexanols from nitroalkenes in the presence of 2′-(diphenylphosphino)biphenyl-2-ol. | ||
Although reaction of nitroalkenes 1 with α-ketoamides 128 furnished the Michael adducts in the presence of Takemoto urea catalyst Cat-4, fluorinated nitroalkene1 (Ar = 4-FC6H4) shows high tendency to overreact with the 1,4-adduct and generate the densely functionalized cyclohexane derivative 129 in a remarkably excellent stereoselectivity of 98% ee (Scheme 39). The reaction proceeded via a Michael–Michael–Henry type of cascade reaction where six stereogenic carbons are created and controlled in one single reaction.64
An elegant procedure for diastereo- and enantioselective synthesis of polysubstituted cyclohexanols 132 is developed by Enders et al. using simple starting materials such as nitroalkenes 38, β-ketoesters 130 and α,β-unsaturated aldehydes 131 (Scheme 40).65 The reaction proceeds via a one-pot consecutive three-component sequence. The reaction started by Michael addition of β-ketoesters to nitroalkenes in the presence of bifunctional thiourea amine catalyst Cat-21, followed by addition of an enal and an achiral secondary amine in stoichiometric amounts at rt after 24 h, and stirring for additional 24 h at room temperature. Consequently, the cyclohexanols 132 containing diverse functional groups such as hydroxy, ester or nitro were obtained in moderate to high yields and excellent diastereo- and enantioselectivites. Interestingly, during this process, up to three new carbon–carbon bonds and six adjacent stereogenic centers, including one quaternary, were created.
 | ||
| Scheme 40 Asymmetric synthesis of cyclohexanols via an organocatalyzed consecutive Michael–Michael–aldol sequence. | ||
Accordingly, Wang et al. reported another highly enatioselective procedure for synthesis of hexasubstituted chiral cyclohexanols 134 via a pseudo-three-component domino Michael–Michael–Henry reaction between aliphatic aldehydes 133 and 2 equiv. of nitroalkenes 38 catalysed by a combination of chiral diphenylprolinol trimethylsilyl ether Cat-5 and a chiral quinine-derived thiourea Cat-17.66 The products obtained in moderate to good yields and diastereoselectivities of up to 60% de, combined with excellent enantioselectivities ranging from 96 to >99% ee (Scheme 41). The authors proposed that the first Michael reaction was induced by the proline-derived catalyst, while the second Michael reaction was catalysed by the chiral thiourea catalyst.
 | ||
| Scheme 41 Domino Michael–Michael–Henry reaction for synthesis of hexasubstituted chiral cyclohexanols. | ||
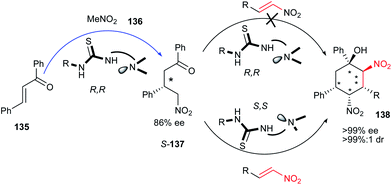
Although the bifunctional thioureas have been widely used in organic transformations, Holczbauer et al. reported that these compounds can have synthetic limitations in multicomponent domino reactions. For this purpose, they have studied the reaction of enantioenriched S-137, which was formed by Michael addition of nitromethane 136 to chalcone 135, with nitroalkenes 38 under catalytic amount of thiourea (R,R)-Cat-22.67 They have found that no products were detected after 1 weak. The authors supposed that the combination of the chiral catalyst (R,R)-Cat-22 and its chiral product S-137 generates a mismatched pair with a sufficiently high barrier to the succeeding intermolecular reaction; thus, the subsequent organocatalytic step becomes kinetically unfavorable. Accordingly, when pseudoenantiomer of Cat-22 was used in the second step, the cyclohexane derivatives 138 were obtained with excellent enantio- (>98% ee) and diastereoselectivity (>93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 dr) (Fig. 4). Due to these observations, this catalytic system was applied for synthesis of a diversity of cyclohexane derivatives bearing five stereogenic centers from the reaction of enantioenriched-137 and rac-137 with aliphatic, aromatic and heteroaromatic nitroalkenes. As expected, in the case of rac-137, the matched reactions were more rapid and highly selective kinetic resolutions were achieved providing the cyclohexane products with high enantio- and diastereoselectivity. With using the catalysts in the reverse order, 139 can be obtained as the enantiomer of 138 with same stereoselectivities (Scheme 42).
7 dr) (Fig. 4). Due to these observations, this catalytic system was applied for synthesis of a diversity of cyclohexane derivatives bearing five stereogenic centers from the reaction of enantioenriched-137 and rac-137 with aliphatic, aromatic and heteroaromatic nitroalkenes. As expected, in the case of rac-137, the matched reactions were more rapid and highly selective kinetic resolutions were achieved providing the cyclohexane products with high enantio- and diastereoselectivity. With using the catalysts in the reverse order, 139 can be obtained as the enantiomer of 138 with same stereoselectivities (Scheme 42).
 | ||
| Scheme 42 Synthesis of both enantiomers of cyclohexane derivatives with proper selection of catalyst. | ||
Highly functionalized cyclohexanols with four stereogenic centers were synthesized with a one-pot three-component reaction of dimethylmalonate 140, an α,β-unsaturated aldehyde 34 and a nitroalkene 1 via Michael–Michael–aldol sequence involving two different organocatalysts Cat-17 and Cat-24 (Scheme 43).68 This catalyst system afford products 141 in 45–87% yield, up to 9.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8
1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and up to >99% ee. When (R)-Cat-24 was combined with (S)-Cat-17, the epi-141 was obtained in 47–69% yield, 7.1
1 dr and up to >99% ee. When (R)-Cat-24 was combined with (S)-Cat-17, the epi-141 was obtained in 47–69% yield, 7.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8
1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, and up to >99% ee. The authors assumed that the first bifunctional base/Brønsted acid catalyst activates both the malonate esters and nitroalkene leading to stereoselective formation of Michael adducts 142. The thus-formed Michael adduct 142 subsequently undergoes a regioselective nitro-Michael reaction to the enal 143 under iminium activation with secondary amine (S)-Cat-24 to afford intermediate 144, followed by a base-promoted aldol cyclization to generate the desired cyclohexanols 141. Control experiments suggested that the iminium catalyzed nitro-Michael addition is also base promoted, and therefore that both catalysts work cooperatively.
1 dr, and up to >99% ee. The authors assumed that the first bifunctional base/Brønsted acid catalyst activates both the malonate esters and nitroalkene leading to stereoselective formation of Michael adducts 142. The thus-formed Michael adduct 142 subsequently undergoes a regioselective nitro-Michael reaction to the enal 143 under iminium activation with secondary amine (S)-Cat-24 to afford intermediate 144, followed by a base-promoted aldol cyclization to generate the desired cyclohexanols 141. Control experiments suggested that the iminium catalyzed nitro-Michael addition is also base promoted, and therefore that both catalysts work cooperatively.
 | ||
| Scheme 43 Synthesis of polysubstituted cyclohexanols 141 and its epimer via controlled catalyst system; TES = triethylsilyl. | ||
Functionalized six-membered spirocyclic compounds 148 and 149 were prepared by Chen et al. via a one-pot cascade protocol using (E)-5-nitro-6-aryl-hex-5-en-2-one 145 and dinucleophilic components such as indane 1,3-diones 146, oxindoles or coumaranone 147 (Scheme 44).69 The products were obtained in good to high yields (30–84%) and high to excellent diastereoselectivities (up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). The optimal conditions for this reaction involved the use of a dinucleophile (0.6 mmol) and 145 (0.5 mmol) in the presence of DABCO (0.5 mmol) in EtOH at 80 °C. Aliphatic nitroalkenes were examined in this protocol without any results. The enantioselective version of this protocol was also investigated using 10 mol% of quinine derived thiourea catalyst Cat-17 in the reaction of coumaranone and 145 to give the corresponding product in 79% enantioselectivity, and 9
1 dr). The optimal conditions for this reaction involved the use of a dinucleophile (0.6 mmol) and 145 (0.5 mmol) in the presence of DABCO (0.5 mmol) in EtOH at 80 °C. Aliphatic nitroalkenes were examined in this protocol without any results. The enantioselective version of this protocol was also investigated using 10 mol% of quinine derived thiourea catalyst Cat-17 in the reaction of coumaranone and 145 to give the corresponding product in 79% enantioselectivity, and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric ratio.
1 diastereomeric ratio.
An asymmetric synthesis of functionalized 1,2,3,4-tetrahydronaphthalen-1-ols 151 bearing four stereogenic centers is reported by Enders et al. using 2-(nitromethyl)benzaldehyde 150 and nitroalkenes 1 in the presence of bifunctional quinine-based squaramide organocatalyst Cat-25 (5 mol%) in CHCl3 at −20 °C (Scheme 45).70 The products were obtained in moderate to very good yields (25–84%), high diastereomeric ratios (dr > 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) after crystallization and good to excellent enantioselectivities (63–99% ee). Among the several hydrogen-bonding thiourea and squaramide organocatalysts examined for this transformation, only Cat-25 gave the best results by considering the yield and stereoselectivity. The stereochemistry of products was examined as (1R,2S,3R,4R). They also proved that nitroolefins with a substituent in meta-position afforded much better ee than their para-counterparts. α,β-Substituted nitroalkenes are not suitable Michael acceptors for this protocol. It is surprising that the Z-nitroalkenes gave similar results in this protocol, which may be assumed that a slow (E/Z) isomerization of nitrostyrene can occur under the reaction conditions. Surprisingly, the ortho-methoxy substituted 150 gave the 4-epimer of 151 under similar conditions. The authors also confirmed that by using γ-nitroaldehydes and ketones, secondary and tertiary cyclohexanols bearing four stereogenic centers can be achieved with this Michael–Henry domino reaction.
5) after crystallization and good to excellent enantioselectivities (63–99% ee). Among the several hydrogen-bonding thiourea and squaramide organocatalysts examined for this transformation, only Cat-25 gave the best results by considering the yield and stereoselectivity. The stereochemistry of products was examined as (1R,2S,3R,4R). They also proved that nitroolefins with a substituent in meta-position afforded much better ee than their para-counterparts. α,β-Substituted nitroalkenes are not suitable Michael acceptors for this protocol. It is surprising that the Z-nitroalkenes gave similar results in this protocol, which may be assumed that a slow (E/Z) isomerization of nitrostyrene can occur under the reaction conditions. Surprisingly, the ortho-methoxy substituted 150 gave the 4-epimer of 151 under similar conditions. The authors also confirmed that by using γ-nitroaldehydes and ketones, secondary and tertiary cyclohexanols bearing four stereogenic centers can be achieved with this Michael–Henry domino reaction.
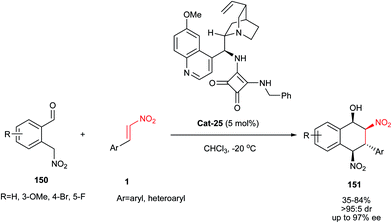 | ||
| Scheme 45 Bifunctional quinine-based squaramide catalyzed synthesis of functionalized 1,2,3,4-tetrahydronaphthalen-1-ols 151. | ||
In 2011, Xu and co-workershave shown a new route for synthesis of complex molecules 154 containing cyclohexanol rings via a sequential Michael–Michael–Michael–aldol cascade mechanism.71 Reactions of acyclic enones 152 and functionalized nitroolefins 153 promoted by Cat-26 and 4-nitrobenzoic acid afforded the optically active cyclohexanones, which were subsequently engaged in a tetrabutylammonium fluoride mediated intramolecular Michael–aldol domino transformation to access the complex tetracyclic structures 154 in good yields and excellent stereoselectivities (Scheme 46). This domino approach allows the formation and control of six stereogenic centers, two of which are quaternary.
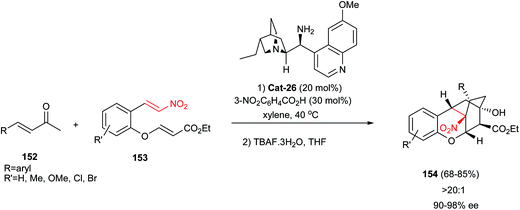 | ||
| Scheme 46 Synthesis of bicyclic [2.2.2] ring systems via a one-pot Michael–Michael–Michael–aldol approach. | ||
Finally, Zou et al. described that reaction of nitroalkene C-glycosides 155 and 157 with variety of primary amines under basic conditions followed by reduction with NaBH3CN afforded the highly functionalized morphan derivatives 156 and 158a containing cyclohexanol ring as major products (Scheme 47).72 In this reaction, three (two C–N and one C–C) new bonds and four stereogenic centers were created in a one-pot procedure under very mild conditions. The proposed mechanism by the authors revealed that the reaction proceeded via β-elimination/Michael addition cascade, followed by Michael addition of theamine and reduction of the generated intramolecular enamination product. In addition, it is notable that the nitroalkene C-glycosides 155 and 157 were prepared from the respective allyl-C-glycoside intermediates of D-ribose and D-arabinose by performing 2′-oxidation prior to β-elimination at C5.
5.3. Synthesis of cyclohexanone derivatives
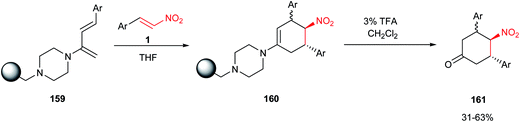
An efficient procedure for synthesis of 3,4,5-trisubstituted cyclohexanones 161 was reported by Hird et al.73 via the Diels–Alder reaction of a resin-bound 2-piperazinylbutadiene74 159 and trans-2-nitrostyrenes 1. Hydrolysis of adducts 160 with 3% TFA in CH2Cl2 afforded the products in good yield and purity (Scheme 48).Feng et al. reported a facile and efficient protocol for synthesis of multifunctionalized tetrahydroindan derivatives 163 with four stereocenters via two sequential Michael reactions between cyclic γ,δ-unsaturated-β-ketoester 162 and nitroalkenes 38 (Scheme 49).75 The reaction promoted by 0.5–2 mol% of cinchona alkaloid Cat-27 and then with 1 equiv. of tetramethylguanidine (TMG) for cyclization. The corresponding products were obtained in high yields (up to 99% yield) with excellent enantioselectivities (95–99% ee) as well as diastereoselectivities (up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) even on a gram scale. Aliphatic nitroalkenes gave lower yield in longer reaction time compare to aromatic nitroalkenes, but with the same stereoselectivities. The authors described that the free phenolic hydroxyl group on the catalyst play a crucial role in activating and fixing substrate to accomplish good stereoinduction. X-ray diffraction analysis shows that the ring junction in product 163 was in favor of cis-fused isomer, and the R-group and nitro group oriented also on the same side.
1 dr) even on a gram scale. Aliphatic nitroalkenes gave lower yield in longer reaction time compare to aromatic nitroalkenes, but with the same stereoselectivities. The authors described that the free phenolic hydroxyl group on the catalyst play a crucial role in activating and fixing substrate to accomplish good stereoinduction. X-ray diffraction analysis shows that the ring junction in product 163 was in favor of cis-fused isomer, and the R-group and nitro group oriented also on the same side.
A double Michael addition reaction between nitrostyrenes 1 and ϒ,δ-unsaturated β-ketoesters 164 is reported by Hoashi et al. to give highly functionalized nitrocyclohexanones 165 in good yields (Scheme 50).76 The Takemoto thiourea organocatalyst Cat-4 was employed to initiate the first Michael addition. Then, the addition of a stronger base (KOH or tetramethylguanidine) is required to achieve cyclization adducts with three contiguous asymmetric carbon atoms. The high stereoselectivity of the second Michael addition can be explained invoking the transition state A energetically more favorable than the transition state B. The relative configurations of major diastereomers were determinedas 3,4-trans-4,5-cis by 1H NMR analysis. This reaction was further applied as key step for the total synthesis of (−)-epibatidine, a potent nicotinic acetyl choline receptor agonist and a non-opiate analgesic approximately 200-fold more potent than morphine.
Accordingly, Namboothiri et al. investigated the reaction of curcumins 166 with nitroalkenes 38 for synthesis of functionalized cyclohexanones 167 possessing three contiguous chiral centers in high to excellent yields and complete diastereoselectivities (Scheme 51).77 The reaction proceeded via inter–intramolecular double Michael addition cascade in the presence of K2CO3 in THF/H2O (7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature as optimal conditions. Other bases such as DBU and KOtBu in different organic solvents also provided the products, but in moderate yield and selectivity. Aromatic, heteroaromatic and aliphatic nitroolefins react equally well in this protocol. Not only naturally occurring curcumin, but also unsubstituted analog and heteroaromatic analog afford the corresponding products in good yield and selectivity. The role of water is considered as protonating agent in double Michael adducts to prevent the retro-Michael reaction, resulting in high diastereoselectivity in the cyclohexanone formation.
1) at room temperature as optimal conditions. Other bases such as DBU and KOtBu in different organic solvents also provided the products, but in moderate yield and selectivity. Aromatic, heteroaromatic and aliphatic nitroolefins react equally well in this protocol. Not only naturally occurring curcumin, but also unsubstituted analog and heteroaromatic analog afford the corresponding products in good yield and selectivity. The role of water is considered as protonating agent in double Michael adducts to prevent the retro-Michael reaction, resulting in high diastereoselectivity in the cyclohexanone formation.
In addition, the asymmetric version of this reaction was carried out with the same group using dihydrocinchonine-thiourea organocatalyst Cat-28 and K2CO3 to give the 4-nitro-cyclohexanones 167 in high yields, diastereoselectivities (up to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) and enantioselectivities (up to 91%).78 The catalyst activates the curcumin by abstracting the hydrogen of active methylene and enolate formation, and also nitroolefins by hydrogen bonding (Scheme 52).
10) and enantioselectivities (up to 91%).78 The catalyst activates the curcumin by abstracting the hydrogen of active methylene and enolate formation, and also nitroolefins by hydrogen bonding (Scheme 52).
 | ||
| Scheme 52 Asymmetric synthesis of nitrocyclohexanones using dihydrocinchonine-thiourea organocatalyst Cat-28. | ||
In 2009, Melchiorre and co-workers showed that the reaction between acyclic enones 168 and functionalized nitroolefins 38 in the presence of 9-amino-9-deoxy-epi-hydroquinine Cat-26 and 2-fluorobenzoic acid as co-catalyst afforded the cyclohexanones 169 bearing up to four contiguous stereogenic centers (Scheme 53).79 The nature of the co-catalyst was important for the outcome of the reaction as both the rate and the diastereoselectivity could be enhanced when salicylic acid was applied instead of 2-fluorobenzoic acid, albeit with a slight decrease in the enantioselectivity.48 According to the proposed mechanism, the reaction proceeds via a stepwise Michael–Michael addition sequence in which the catalyst activates the substrates through an enamine–iminium process. The selective formation of the trans-169 product nicely complements the methodology developed by Barbas, who obtained, under similar conditions, the cis-169 products through a [4 + 2] cycloaddition.80
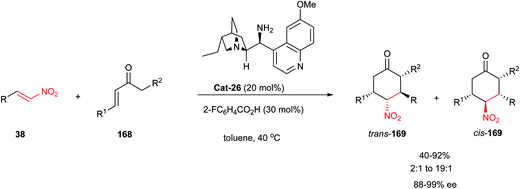 | ||
| Scheme 53 Cyclohexanones bearing up to four stereogenic centers by a domino Michael–Michael transformation. | ||
The development by Sun and co-workers in 2009 of an enantioselective organocatalyzed Michael–elimination–proton transfer–Michael domino reaction allows the formation of functionalized bicyclo[3.3.1]nonane systems 172 with good to excellent diastereo- and enantioselectivities (Scheme 54).81 In the paper, the authors completed their methodology with theoretical calculations justifying the choice of the organocatalyst Cat-29, which allowed them to propose a transition state that explains the observed stereoselectivities.
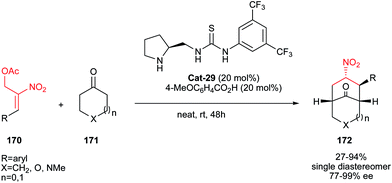 | ||
| Scheme 54 Cascade Michael–elimination–proton transfer–Michael for synthesis of functionalized bicyclo[3.3.1]nonanes. | ||
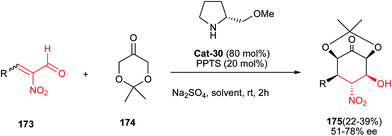
Finally, synthesis of highly oxygenated nitrocyclohexanes 175 bearing five stereogenic centers is reported by Alonso and co-workers in 2012. They described that the Michael–aldol cascade reactions between α-nitroenals 173 and 2,2-dimethyl-1,3-dioxan-5-one 174 is efficiently promoted by O-methylprolinol Cat-30 to give the corresponding products 175 with moderate yields and stereoselectivities (Scheme 55).82 In addition, this strategy was applied by the authors for the total synthesis of (+)-pancratistatin and (−)-tetrodotoxinin natural products.
5.4. Synthesis of cyclohexene derivatives
Cycloaddition reaction of resin-bound nitroalkene 176, simply prepared via a microwave assisted Knoevenagel reaction of resin-bound nitroacetic acid (Wang resin; p-benzyloxybenzyl alcohol functionalized polystyrene resin, 100–200 mesh) and benzaldehyde, with 2,3-dimethylbutadiene 177 under 15k bar pressure afforded the corresponding resin-bound nitrocyclohexene 178 as outlined in Scheme 56.83 Reduction of 178 with LiAlH4 or SnCl2/LiAlH4 afforded substituted cis cyclohexene derivatives 179 or 180 respectively.Very recently, Prajapati et al. reported a mild, one-pot and efficient procedure for synthesis of a series of tetrahydroquinazolinedione frameworks 182 via a [4 + 2] cycloaddition strategy without using any catalyst. They described that reaction of 6-[2-(dimethylamino) vinyl]-1,3-dimethyluracil 181 with an equimolar amount of nitrostyrene 1 in refluxing chloroform for six hours gave, without elimination of dimethylamine, the corresponding tetrahydroquinazolinedione derivatives 182 as the only product in high yields (Scheme 57).84 This reaction did not proceed with aliphatic nitroalkenes.
The use of the nitroalkene moiety as a heterodiene in formal [4 + 2] cyclizations leading to nitronates has been extensively exploited by Denmark and coworkers as an effective strategy for the synthesis of alkaloids.3a,85 Nevertheless, there are surprisingly few examples of intramolecular Diels–Alder processes (IMDA) which describe the use of nitroalkenes as dienophilic components.86
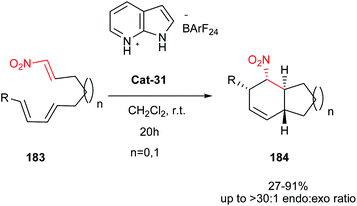
In this context, IMDA cyclizations of (E)-1-nitro-1,6,8-nonatrienes and (E)-1-nitro-1,7,9-decatrienes 183 is investigated by Takenaka and Aguado in the presence of pyridinium salt Cat-31 as a Bronsted acid catalyst (Scheme 58).87 Under these conditions, the reaction proceeds at room temperature to give the endo selective products 184. Also the present study demonstrates that the Brønsted acid catalysis is an effective strategy for increasing the rate and diastereoselectivity of this class IMDA reactions for which Lewis acid catalysis has proved difficult.
Diels–Alder reaction of (E)-2-methyl-1,3-pentadiene 185 with in situ prepared nitroalkene 187, which can be simply prepared by the reaction of phenylsulfonylnitromethane 188 with formaldehyde, gave 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 diastereomeric mixture of cycloadducts 189a and 189b in 61% yield (Scheme 59).88 The configuration of the major isomer was assigned as (R,R)-189a. By using cyclopentadiene 186 as diene, 190 was obtained as main product with the preferred endo-placement of the nitro group.
20 diastereomeric mixture of cycloadducts 189a and 189b in 61% yield (Scheme 59).88 The configuration of the major isomer was assigned as (R,R)-189a. By using cyclopentadiene 186 as diene, 190 was obtained as main product with the preferred endo-placement of the nitro group.
Diels–Alder reaction of (Z)-1,1,4-trichloro-2,4-dinitrobuta-1,3-diene 191 with dienes such as isoprene 192, 2,3-dimethyl 1,3-butadiene 177 and cyclopentadiene 186 in a sealed ampule at 60–85 °C within 40–60 hours provided the corresponding cyclohexenes 193a/b, 194 and bicyclic products 195 in 25, 37 and 60% isolated yield, respectively (Scheme 60).89 Interestingly, the additional α-nitro-β,β-dichloro vinyl group shows no such reactivity with respect to Diels–Alder reactions. Notably, the low yields of the products are due to the polymerization of the starting materials during the reaction conditions. The β,β-dichloro nitroalkene moiety in the structure of products were subsequently reacted with appropriate aromatic N,O- or N,N-bisnucleophiles 196 to give the benzoxazole or benzimidazole derivatives 197.
Although the β-nitrostyrenes have been widely employed in Diels–Alder reaction as dienophiles, they are remained inert toward trienamine 198, even at elevated temperature (80 °C). To overcome this limitation, Chen et al. described that by introducing an alkyl or aryl group into the skeleton of 2,4-dienals, which may raise the HOMO-energy of the resulting trienamine intermediates 199 (Scheme 61), the normal-electron-demand Diels–Alder (NEDDA) reaction can be carried out with nitroolefins. The scope for both substrates is completely investigated, and a number of densely substituted cyclohexenes 200, including bicyclic ring frameworks have been constructed with excellent enantioselectivity and good diastereocontrol in the presence of catalytic amount of Jørgensen–Hayashi catalyst Cat-5. Unexpectedly, this NEDDA reaction of nitroolefins exhibited abnormal exo-selectivity which might be due to electrostatic repulsion between the π electrons of the trienamine motif and the nitro group, as depicted in model A in Scheme 62.90
The same strategy was applied by Melchiorre et al. for construction of polycyclic heteroaromatic compounds 203. They have shown that reaction of 3-(2-methyl-indol-3-yl)acrylaldehyde 201 with nitroalkenes 38 promoted by Jørgensen–Hayashi catalyst Cat-5 gave adducts 203 with high chemical yields and excellent stereoselectivities via DA reaction of in situ generated indole-2,3-quinodimethane 202 (Scheme 63).91
Another approach for synthesis of these products with different configurations was reported by Wang et al. via a double Michael addition–aromatization cascade reaction of 2-propenylindoles 204 and nitroolefins 1 in the presence of chiral bis-sulfonamide catalyst Cat-32 and an acid additive (Scheme 64).92 This strategy provides functionalized cyclohexene rings fused to indole derivatives 205 in moderate to good yields (42–86%) with good to excellent diastereoselectivities (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20–99
20–99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and good enantioselectivities (82–92% ee). The authors proposed that hydrogen bonding activation of nitroalkenes with chiral catalyst plays an important role in asymmetric preparation of products.
1) and good enantioselectivities (82–92% ee). The authors proposed that hydrogen bonding activation of nitroalkenes with chiral catalyst plays an important role in asymmetric preparation of products.
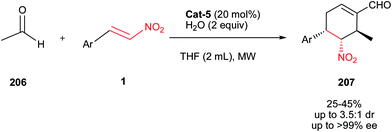
Asymmetric synthesis of trisubstituted cyclohexene carbaldehyde 207 is reported by Enders et al. via a microwave-assisted, organocatalytic quadruple domino Michael–Henry condensation–Michael–aldol condensation reaction employing acetaldehyde 206 (excess) and nitroalkenes 1 as substrates in moderate to good yields (25–45%) and high enantioselectivities (ee = 89 to >99%) as shown in Scheme 65.93 They have shown that the electronic features and the position of the substituent on the aromatic ring have significant influence on the reaction time (from 5 hours up to 12 hours) and the diastereoselectivity of the reaction (from 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 down to 1.4
1 down to 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr), but have no influence on the enantioselectivity. However, the method is limited by formation of an appreciable quantity of the C-5 epimer as the minor isomer (3.5–1.4
1 dr), but have no influence on the enantioselectivity. However, the method is limited by formation of an appreciable quantity of the C-5 epimer as the minor isomer (3.5–1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In is notable that the presence of at least two equivalents of water in reaction is crucial for completion. In the absence of water, formation of the aldehyde 207 was not observed.
1). In is notable that the presence of at least two equivalents of water in reaction is crucial for completion. In the absence of water, formation of the aldehyde 207 was not observed.
A proposed mechanism by the authors is given in Scheme 66. The reaction starts with the Michael addition of enamine of acetaldehyde 206 to nitroalkene 1 to generate the nitroalkane 208. In the next step, a Henry reaction between the resulting nitroalkane 208 and a second acetaldehyde followed by elimination of water provides the newly formed nitroalkene 209. Enamine activation of a third acetaldehyde and Michael addition of this to 209 affords the dialdehyde 210. Finally, intramolecular aldol condensation reaction via enamine activation, followed by hydrolysis, affords the product 207 and regenerates the catalyst Cat-5 for the next catalytic cycle. The proposed mechanism was successfully supported by ESI-MS measurements and other experiments.
In addition, the same group reported another one-pot multicomponent process for synthesis of substituted cyclohexenecarbaldehyde rings using indoles 211, acrolein 212 (2 equiv.), and nitroalkenes 38 as substrates (Scheme 67).94 The reaction proceeds via a quadruple domino Friedel–Craftstype–Michael–Michael–aldol condensation catalyzed by Cat-5 to afford 3-(cyclohexenylmethyl)-indoles 213 bearing three vicinal stereogenic centers in moderate to good yield (23–84%) and excellent stereoselectivity (91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 → 95
9 → 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, 94 → 99% ee). A proposed mechanism by the authors revealed that the iminium–enamine tautomerization plays an important role in the stereoselective formation of products.
5 dr, 94 → 99% ee). A proposed mechanism by the authors revealed that the iminium–enamine tautomerization plays an important role in the stereoselective formation of products.
 | ||
| Scheme 67 Organocatalytic one-pot multi-component quadruple domino Friedel–Crafts-type–Michael–Michael–aldol condensation. | ||
In continuation, they have also expanded this strategy for other electron-rich arenes such as m-anisidine 214 (Scheme 68).95 So, the corresponding products 215 were obtained in 30–59% yields and excellent stereoselectivities (dr > 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5%, ee 94 → 99%). The optimum conditions for this reaction include the use of nitroalkene 1 (2 mmol), Cat-5 (20 mol%), benzoic acid (20 mol%), excess amount of the m-anisidine 214 (8 mmol) in chloroform (5 mL) under argon in a Schlenk-flask at 30 °C with slowly addition of acrolein 212 solution (14 mmol) in chloroform (5 mL) over 7 h. Nitroalkenes with electron-donating and -withdrawing group on the phenyl ring and heteroaromatic nitroalkenes were tolerated with good yield and excellent enantioselectivity. Furthermore, a one-pot four-component version of this reaction using nitrostyrene 1 (Ar = Ph), crotonaldehyde 212, cinnamaldehyde 34 and the m-anisidine 214 (R = Me) was also investigated to give the corresponding cyclohexenecarbaldehydes 216 with an additional stereocenter (Scheme 69). The reaction resulted in a moderate yield (37%, dr > 95
5%, ee 94 → 99%). The optimum conditions for this reaction include the use of nitroalkene 1 (2 mmol), Cat-5 (20 mol%), benzoic acid (20 mol%), excess amount of the m-anisidine 214 (8 mmol) in chloroform (5 mL) under argon in a Schlenk-flask at 30 °C with slowly addition of acrolein 212 solution (14 mmol) in chloroform (5 mL) over 7 h. Nitroalkenes with electron-donating and -withdrawing group on the phenyl ring and heteroaromatic nitroalkenes were tolerated with good yield and excellent enantioselectivity. Furthermore, a one-pot four-component version of this reaction using nitrostyrene 1 (Ar = Ph), crotonaldehyde 212, cinnamaldehyde 34 and the m-anisidine 214 (R = Me) was also investigated to give the corresponding cyclohexenecarbaldehydes 216 with an additional stereocenter (Scheme 69). The reaction resulted in a moderate yield (37%, dr > 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and excellent enantioselectivity (ee >99%).
5) and excellent enantioselectivity (ee >99%).
Another one-pot four-component asymmetric synthesis of alkoxy-substituted cyclohexenecarbaldehydes 218 is reported by Zhang et al. in 2009 from nitroalkenes 1, alcohols 217 and two equiv. of acrolein 212 (Scheme 70). This protocol initiated by the oxa-Michael addition of alcohols onto acrolein to afford good to high yields of products with excellent enantioselectivity in the presence of a catalytic amount of Jorgensen–Hayashi catalyst Cat-5 (5 mol%) and benzoic acid (25 mol%).96
Tetrasubstituted cyclohexenecarbaldehydes 191 has been prepared by Enders et al. with the reaction of a linear aldehyde 118, a nitroalkene 38, and an α,β-unsaturated aldehyde 219 (Scheme 71).97 In the presence of Cat-5, the reaction proceeds via a Michael–Michael–aldol sequence resulting in enantioselective formation of two diastereomers 220a and 220b bearing four stereocenters (The diastereomeric ratios of 220a to the minor 5-epimer 220b vary from 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 to 99
32 to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The main diastereomer can be easily isolated with >99% diastereo and enantioselectivities after column chromatography of the reaction mixture.
1). The main diastereomer can be easily isolated with >99% diastereo and enantioselectivities after column chromatography of the reaction mixture.
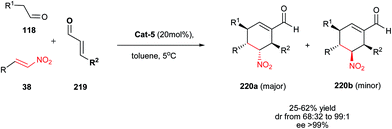 | ||
| Scheme 71 Asymmetric synthesis of tetrasubstituted cyclohexenecarbaldehydes from nitroalkenes, aldehydes and α,β-unsaturated aldehydes. | ||
They also described that the products 220a are suitable intermediates for synthesis of complex molecules. For this purpose, they demonstrated that the highly functionalized chiral tricyclic carbaldehydes 221a/b can be obtained using (5E,7E)-nona-5,7-dienal (n = 0) and (6E,8E)-deca-6,8-dienal (n = 1) instead of 219 in the same strategy, followed by intramolecular Diels–Alder reaction in the presence of dimethylaluminium chloride (Me2AlCl). This process led to the formation of five carbon–carbon bonds and eight stereogenic centers with complete enantiocontrol. The reaction was amenable to various substrates and products containing 5- or 6-membered rings 221a/b (n = 0, 1) which could be synthesized in good yields (35–56%) and diastereomeric ratios (5.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–15
1–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) with excellent levels of asymmetric induction (≥99% ee) (Scheme 72). The present method also gives rise to chiral motifs that exist in diterpenoid natural products such as hainanolides and amphilectanes.98 The intermediate 220a was also applied as an efficient starting material for construction of 3-substituted hexahydro-3H-isochromenes 222 via hetero-Diels-Alder reaction and thiadecalines 223 via a sulfa-Michael addition reaction.99
1 dr) with excellent levels of asymmetric induction (≥99% ee) (Scheme 72). The present method also gives rise to chiral motifs that exist in diterpenoid natural products such as hainanolides and amphilectanes.98 The intermediate 220a was also applied as an efficient starting material for construction of 3-substituted hexahydro-3H-isochromenes 222 via hetero-Diels-Alder reaction and thiadecalines 223 via a sulfa-Michael addition reaction.99
In addition, an alternative protocol for synthesis of products 220a from the same starting material is reported by Wang et al. via an environmentally benign procedure using dithienylprolinol silyl ether Cat-33 (10 mol%), N-Boc-D-phenylglycine (20 mol%) and SDBS (20 mol%) in water (Scheme 73). High diastereoselectivity (up to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr) and excellent enantioselectivity (>99 ee) was achieved in this protocol.100
10 dr) and excellent enantioselectivity (>99 ee) was achieved in this protocol.100
Reuping and co-workers described that it is possible to chemoselectively reduce an enal into the corresponding saturated aldehyde in the presence of a nitroolefin using Cat-5 as catalyst and Hantzsch dihydropyridine 224 as reducing agent. With this result in hand, they demonstrated an asymmetric approach for synthesis of pentasubstituted cyclohexenes 225 from a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of simple enals 34 and nitroolefins 1 via a hydrogenation–Michael–Michael–aldol quadruple domino sequence (Scheme 74).101 Indeed, they transformed a pseudo three-component reaction into a real three-component domino sequence via in situ reduction of an equivalent of the enal 34 into an aldehyde, which then stereoselectively added to the nitroalkene through enamine activation. Consequently, cyclohexenes 225 containing four contiguous chiral centers were obtained as single diastereomers in moderate to good yields and excellent enantioselectivities.
1 mixture of simple enals 34 and nitroolefins 1 via a hydrogenation–Michael–Michael–aldol quadruple domino sequence (Scheme 74).101 Indeed, they transformed a pseudo three-component reaction into a real three-component domino sequence via in situ reduction of an equivalent of the enal 34 into an aldehyde, which then stereoselectively added to the nitroalkene through enamine activation. Consequently, cyclohexenes 225 containing four contiguous chiral centers were obtained as single diastereomers in moderate to good yields and excellent enantioselectivities.
Penta-substituted cyclohexenes 227 containing four adjacent stereogenic centers were synthesized by Enders and co-workers using a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 3-phenyl propanal 226 and nitrostyrenes 1 in the presence of Cat-5 and IBX.102 Their protocol is based on the possible generation of both a Michael donor and a Michael acceptor from the same aldehyde; indeed, they have converted a two-component reaction to a three-component reaction via the in situ oxidation of the enamine of the additional equivalent of 3-phenyl propanal 226 to conjugated iminium with IBX in the presence of Cat-5. So, this sequential oxidation–Michael–Michael–aldol reaction afforded the poly-functionalized cyclohexenes 227 with good to excellent diastereoselectivities and excellent enantioselectivities (Scheme 75).
1 mixture of 3-phenyl propanal 226 and nitrostyrenes 1 in the presence of Cat-5 and IBX.102 Their protocol is based on the possible generation of both a Michael donor and a Michael acceptor from the same aldehyde; indeed, they have converted a two-component reaction to a three-component reaction via the in situ oxidation of the enamine of the additional equivalent of 3-phenyl propanal 226 to conjugated iminium with IBX in the presence of Cat-5. So, this sequential oxidation–Michael–Michael–aldol reaction afforded the poly-functionalized cyclohexenes 227 with good to excellent diastereoselectivities and excellent enantioselectivities (Scheme 75).
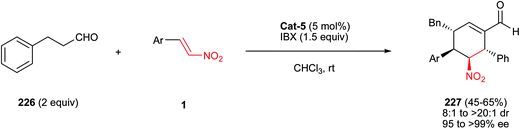 | ||
| Scheme 75 Enantioselective synthesis of polysubstituted cyclohexenes via sequential oxidation–Michael–Michael–aldol reaction. | ||
Indeed, synthesis of cyclohexane derivatives with three all-cis consecutive stereocenters is a persistent challenge in organic synthesis due to steric hindrance. In 2009, Hong and coworkers reported a stereoselective synthesis of all-cis 5-nitro-4,6-diarylcyclohex-1-enecarboxylic esters 228a (major isomer) by an organocatalytic asymmetric Michael–Michael–Wittig cascade reaction of phosphorus ylides 30, nitroolefins 1, and α,β-unsaturated aldehydes 34 (Scheme 76).103 This one-pot operations proceeded via the [1 + 2 + 3] annulations catalyzed by Cat-5 and HOAc as additive with excellent enantioselectivities (up to >99% ee) and good yields.
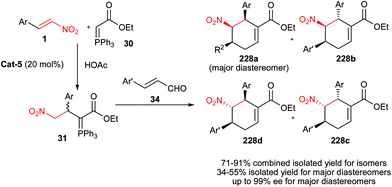 | ||
| Scheme 76 [1 + 2 + 3] Annulations for synthesis of all-cis 5-nitro-4,6-diphenylcyclohex-1-enecarboxylic esters. | ||
Tetrahydronaphthalenes 230 bearing four stereogenic centres were synthesized by Tan et al. via a domino Michael addition/nitrone formation/intramolecular [3 + 2] nitrone-olefin cycloaddition reactions occurring between nitroolefin acrylates 229 and aldehydes 118 upon catalysis with Cat-5 (Scheme 77).104 In this case, the process was performed in aqueous media and afforded a series of biologically significant, heavily functionalised, chiral tetrahydronaphthalenes with good-to-high yields combined with excellent diastereoselectivities (up to 98% de) and enantioselectivities (>99% ee). The authors described that water play dual roles in this reaction as an environmentally benign solvent, and as promoter to improve the reactivity and stereoselectivity.
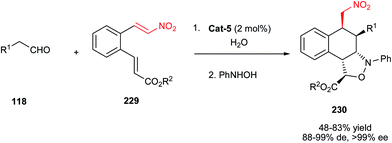 | ||
| Scheme 77 Domino Michael addition/nitrone formation/intramolecular [3 + 2] nitrone-olefin cycloaddition reactions for synthesis of tetrahydronaphthalenes. | ||
Synthesis of cyclohexene carbaldehydes fused to chroman ring 234 were introduced by Hong and co-workers via a three-component quadruple domino reaction from 2-hydroxy-β-nitrostyrene 231 and two different enals 232 and 233 in the presence of Cat-5 (20 mol%) and AcOH (20 mol%) in toluene at room temperature.105 The reaction proceeds via a domino oxa-Michael–Michael–Michael–aldol–dehydration sequence catalyzed by Cat-5 to give the optically active tetrahydro-6H-benzo[c]chromenes 234 in good to high yields and excellent enantioselectivities (Scheme 78). Notably, one C–O and five C–C bonds and five stereogenic centers were created in a one-pot operation. Also, this strategy was applied for the total synthesis of the natural product (+)-conicol.105b
 | ||
| Scheme 78 A one-pot three component asymmetric synthesis of optically active tetrahydro-6H-benzo[c]chromenes. | ||
Hayashi and co-workers have reported a highly efficient synthesis of oseltamivir (Tamiflu™, Fig. 5) 235, a well-known drug for the treatment of influenza, in two ‘one-pot’ sequences starting with a nitroalkene 236.106
The first ‘one-pot’ sequence contains four steps including Michael/Michael/Horner–Wadsworth–Emmons (H–W–E)/thia-Michael reactions as shown in Scheme 79. The asymmetric Michael addition of aldehyde 237 to nitroolefin 236 was catalysed by proline-derived catalyst Cat-5 (1 mol%) and chloroacetic acid as additive in toluene at rt for 6 h to give Michael adduct 238 in 7.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric ratio, 97% ee, and quantitative yield. Nitroaldehyde 238 was then subjected to the second asymmetric Michael addition with vinylphosphonate 239, followed by an intra-molecular Horner–Wadsworth–Emmons reaction in the presence of Cs2CO3 as base to create an equimolar mixture of (5R)-240/(5S)-240, 241 and 242. To improve the yield of reaction toward desired 240, they convert 241 and 242 into 240 via retroaldol and a combination of retroaldol followed by H–W–E, respectively with addition of EtOH to the reaction mixture. With this protocol, complete conversion to a 4.6
1 diastereomeric ratio, 97% ee, and quantitative yield. Nitroaldehyde 238 was then subjected to the second asymmetric Michael addition with vinylphosphonate 239, followed by an intra-molecular Horner–Wadsworth–Emmons reaction in the presence of Cs2CO3 as base to create an equimolar mixture of (5R)-240/(5S)-240, 241 and 242. To improve the yield of reaction toward desired 240, they convert 241 and 242 into 240 via retroaldol and a combination of retroaldol followed by H–W–E, respectively with addition of EtOH to the reaction mixture. With this protocol, complete conversion to a 4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric mixture of (5R)-240 (undesired) and (5S)-240 (desired) was observed. The isomerization of (5R)-240 to (5S)-240 was tried next under basic conditions (K2CO3, Cs2CO3, or TEA) or in contact with silica, but no change in the ratio (K2CO3, Cs2CO3) or a ∼1
1 diastereomeric mixture of (5R)-240 (undesired) and (5S)-240 (desired) was observed. The isomerization of (5R)-240 to (5S)-240 was tried next under basic conditions (K2CO3, Cs2CO3, or TEA) or in contact with silica, but no change in the ratio (K2CO3, Cs2CO3) or a ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture (TEA, silica) was obtained, which seemed to indicate that both isomers had similar thermodynamic stability. According to the theoretical calculations, it was rationalized that the epimerization at C5 would occur more readily in a cyclohexane ring since the epimerizable groups would be in equatorial positions. Thus, the (5R)-240/(5S)-240 mixture was treated with 4-methylthiophenol in EtOH at 15 °C to give Michael-addition product 243 as a single diastereomer in 74% yield from aldehyde 237 and nitroalkene 236 after column chromatogaphy. Under the reaction conditions, the two diastereomers equilibrate to give the desired (5S)-240 predominantly. The Michael addition of 4-methylthiophenol to the acrylate then proceeds stereoselectively and preferentially on the (5S)-isomer to give 243.
1 mixture (TEA, silica) was obtained, which seemed to indicate that both isomers had similar thermodynamic stability. According to the theoretical calculations, it was rationalized that the epimerization at C5 would occur more readily in a cyclohexane ring since the epimerizable groups would be in equatorial positions. Thus, the (5R)-240/(5S)-240 mixture was treated with 4-methylthiophenol in EtOH at 15 °C to give Michael-addition product 243 as a single diastereomer in 74% yield from aldehyde 237 and nitroalkene 236 after column chromatogaphy. Under the reaction conditions, the two diastereomers equilibrate to give the desired (5S)-240 predominantly. The Michael addition of 4-methylthiophenol to the acrylate then proceeds stereoselectively and preferentially on the (5S)-isomer to give 243.
The second ‘one-pot’ sequence is described in Scheme 80. tert-Butyl ester 243 was cleaved with TFA to give carboxylic acid 244, which was converted to acid chloride 245 via treatment with oxalyl chloride and a catalytic amount of DMF. Crude 245 was then reacted with TMSN3 and pyridine in toluene to afford acyl azide 246 which underwent Curtius rearrangement on treatment with Ac2O in HOAc107 at rt to give acetamide 247. Then, reduction of nitro group in 247 with Zn and aqueous 2 N HCl give amine 248 in 86% yield, which was treated with K2CO3 in EtOH to provide oseltamivir free base 235. Hayashi's synthesis of oseltamivir proceeds in 10 steps with an outstanding 60% overall yield.
Also, Ma and co-workers reported another approach for synthesis of oseltamivir starting with nitroalkene 249 (Scheme 81)108 which was prepared by acetylation of (Z)-2-nitroethenanime as the only product.109 Under the reaction conditions, it was hypothesized that 249 would isomerize to 250 to allow the subsequent Michael addition. In the first step, 249 react with aldehyde 237 in the presence of Cat-2 (10 mol%) and benzoic acid (30 mol%) to give Michael-addition product 251 in ∼80% yield and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 syn/anti ratio. Without purification, aldehyde 251 was treated with vinylphosphonate 239 and Cs2CO3 to give cyclohexene 252 as a mixture of epimers at C-5, following a similar strategy as Hayashi and co-workers during their synthesis of oseltamivir. This crude material was then subjected to the reaction with 4-methylthiophenol to give intermediate 247 and its epimer at C-5. The yield for these three steps combined was 54% and 247 was obtained in 96% ee, whose structure was confirmed by X-ray analysis. Reduction of the nitro group with Zn and TMSCl followed by 4-methylthiophenol elimination with K2CO3 in MeOH regenerate the double bond and provided oseltamivir 235 as the free base. This approach only required two separation steps and gave 46% overall yields for five-steps from aldehyde 237 and nitroolefin 249, but additional steps are needed to assemble these two starting materials.
1 syn/anti ratio. Without purification, aldehyde 251 was treated with vinylphosphonate 239 and Cs2CO3 to give cyclohexene 252 as a mixture of epimers at C-5, following a similar strategy as Hayashi and co-workers during their synthesis of oseltamivir. This crude material was then subjected to the reaction with 4-methylthiophenol to give intermediate 247 and its epimer at C-5. The yield for these three steps combined was 54% and 247 was obtained in 96% ee, whose structure was confirmed by X-ray analysis. Reduction of the nitro group with Zn and TMSCl followed by 4-methylthiophenol elimination with K2CO3 in MeOH regenerate the double bond and provided oseltamivir 235 as the free base. This approach only required two separation steps and gave 46% overall yields for five-steps from aldehyde 237 and nitroolefin 249, but additional steps are needed to assemble these two starting materials.
6. Synthesis of seven-membered carbocycles
Carbocyclic seven-membered rings are present in the structure of a number of polycyclic natural products such as phorbol esters,110 guanacastepenes,111 guianolides112 as well as the frondosins113 with considerable medicinal interest. Unlike smaller ring sizes – especially five and six-membered rings – which can be simply synthesized through various cyclization reactions, the construction of seven-membered rings is more challenging. Among the most important useful methods for synthesis of cycloheptanoid natural products, cycloaddition strategies such as the [5 + 2], [4 + 3], [4 + 2 + 1] and [3 + 2 + 2] reactions, Claisen rearrangement, tandem Michael–intramolecular Wittig reactions of a five-membered cyclic phosphonium ylide with α,β-unsaturated esters, sequential oxyanionic 5-exo-dig cyclization/Claisen rearrangement process, and ring expansion of 1-vinylcycloalkanol derivatives are well known.114Another approach for synthesis of fused seven-membered rings is sequential Michael addition/intramolecular annulations reaction. In this context, Liu et al. described an effective pathway for the chemoselective- and regioselective synthesis of tetracyclic indole derivatives containing a seven-membered ring 255 in water with microwave irradiation catalyzed by gold(I) complex Cat-34 and trifluoroactic acid (TFA).115 This procedure presents an operationally simple, rapid and environmentally friendly strategy for preparing complex molecules from simple starting materials (Scheme 82).
 | ||
| Scheme 82 Gold-mediated one-pot domino synthesis of tetracyclic indole derivatives containing a seven-membered ring. | ||
In 2011, Enders et al. have shown that the asymmetric version of this reaction can be performed using a chiral thiourea based organocatalyst Cat-35 and achiral gold complex [Au(PPh3)]NTf2 to give the seven-membered ring containing tetracyclic indole derivatives in good to excellent yields (51–96%) with remarkable enantioselectivities ranging from 97 to 99% ee (Scheme 83).116 The authors assume that the Friedel–Crafts reaction of indole 253 with ortho-alkyne-substituted nitrostyrene 254 catalyzed by Cat-35 furnish the corresponding C3-substituted alkyne intermediate 256, which subsequently undergo a second Friedel–Crafts reaction with gold(I)-activated alkyne to generate a spirocyclic intermediate 257. The latter rearranged through a 1,2-carbon shift to effect an expansion from a six- to a seven-membered ring 258. Subsequent rearomatisation and protodeauration led to the final product 255.
7. Synthesis of decalin derivatives
Decalin structures are important intermediates for the synthesis of biologically active compounds and are widely present in the structure of steroid and terpenoid natural products.117 Different routes have been developed for synthesis of both cis- and trans-decalins.118 Cycloaddition reaction is one of the most important routes for the synthesis of these compounds.119There are surprisingly few examples of IMDA processes which describe the use of nitroalkenes as dienophilic components.120 In this context, IMDA cyclization of (1E,3Z,7E)-1-nitro-deca-1,3,7,9-tetraenes 259 and (1E,7E)-1-nitro-deca-1,7,9-trienes 261 have been investigated by Williams et al. for stereoselective formation of trans-fused decalin products 260a/b and 261a/b. They demonstrated that increasing the steric repulsions in the tethering chain increase the rate of IMDA cyclization as a consequence of a Thorpe–Ingold effect which promote facile stereocontrolled formation of trans-fused decalin products as outline in Scheme 84.
Also, reductive intramolecular Henry reaction of nitroalkenones 263 with Stryker's reagent [Ph3PCuH]6 have been reported by Chiu et al. to produce the two fused rings 264 as outlined in Scheme 85.121 The cis-decalin 264 was obtained as major isomer in most of the cases as a mixture of epimers at the nitro group. In addition, based on the structure of nitroalkenone, intramolecular cyclizations afford both six- and five-membered fused rings. In addition, this approach is applicable for synthesis of substituted nitrocyclopentanols from acyclic niroalkenones.
8. Synthesis of benzene derivatives
Benzene and its derivatives are the most important building blocks in organic chemistry. Polysubstituted benzenes possessing electron-donor and/or acceptor substituents are of considerable interest due to their participations in the structure of a large number of bioactive natural and synthetic compounds,122 and are useful as versatile precursors for asymmetric syntheses,123 and as important substrates for nonlinear optical materials124 and molecular electronic devices.125Although there are numerous procedures for synthesis of benzene and its derivatives, most of them are based on aromatic substitution reactions which introduce a substituent to an existing arene. The most common routes based on this approach involve electrophilic126 or nucleophilic127 substitution, coupling reactions catalyzed by transition metals128 and metalation-functionalization reactions.129 The regioselective synthesis of polysubstituted benzenes starting from acyclic precursors in which the substitution pattern of the final product is dictated by the structures and functional groups of the precursors, have received growing interest in modern organic synthesis due to their short synthetic steps, environmental concern, and the avoidance of regioisomeric problems.130 For this purpose, useful approaches such as [3 + 2 + 1] Dotz reaction of Fisher carbene complexes,131 [4 + 2] cycloaddition of metallacyclopentadienes and alkynes,132 alkyne-cyclobutenone [4 + 2] cyclization,133 transition metal catalyzed [2 + 2 + 2] and [4 + 2] cycloadditions,134,130a [4 + 2] benzannulation of o-alkynyl benzaldehyde and alkyne,135 [3 + 3] cyclocondensation between bielectrophiles and binucleophiles,136 and 1,6-electrocyclization reaction,137 are developed in recent years.
Kuzhaeva et al. described that the substituted arylcarboxylate 266a can be simply prepared in high yields by DA reaction of 3-bromo-3-nitroacrylate 265 with 2,3-dimethyl-1,3-butadiene 177, followed by dehydrohalogenation reaction as shown in Scheme 86.138
It is difficult to introduce amino, nitro, and nitrile group to the aromatic rings in one step. In this context, an efficient, simple and environmentally benign procedure for synthesis of potentially interesting 3-aryl-2,6-dicyano-5-methylanilines 268 is reported by Adib et al. via the reactions between nitrostyrenes 1 and excess malononitrile 267 in the presence of sodium carbonate in 80% ethanol at room temperature (Scheme 87).139 The main advantages of this method are good yields of the products, relatively short reaction times, a straightforward purification process, mild reaction conditions and the use of cheap and readily available starting materials. The reaction is also possible with nitroalkenes derived from nitroethane instead of nitromethane, but in lower yield. They also claimed that the synthesis of such highly substituted benzene derivatives has not been achieved by transition-metal-catalyzed reactions.
In general, it is difficult to synthesise a fully substituted aromatic ring. Accordingly, Xue et al. developed a one-pot two-step protocol for synthesis of polysubstituted benzene derivatives 270 from nitroalkenes 38 and vinyl malononitriles 269, which were simply prepared from the corresponding ketones via a Knoevenagel reaction (Scheme 88).140 The first step of this reaction is catalyzed by Et3N to afford the vinylogous Michael addition adducts. This intermediate undergoes sequential tandem reaction in the presence of 100 mol% NaOEt in refluxing CH3CN under air to give polysubstituted benzene derivative in high yield. A wide range of nitroolefins bearing aliphatic, aryl, and heteroaryl groups are suitable for this transformation.
In another report, Su et al. demonstrated a simple one-pot procedure for synthesis of fully substituted arylnitriles 270 and arylcarboxylates 272 via cyclocondensation of activated α-methylenealkenes such as vinyl malononitriles 269 and ethyl vinyl cyanoacetates 271 with nitroolefins 38 using Cu(OTf)2/Et3N as an efficient catalytic system (Scheme 89).141 The presence of a base for progress of reaction is mandatory. Other metal triflate/Et3N catalyst systems, such as Zn(OTf)2/Et3N, Sc(OTf)3/Et3N, and Mg(OTf)2/Et3N, were also examined for this transformation; and only Sc(OTf)3/Et3N showed good activity.
Fully substituted diaminobenzonitriles 274 were prepared by Sadek et al. via a simple and efficient procedure using 1,1,3-tricyano-2-aminopropionitrile 273 and nitroolefins 1 in the presence of three drops of piperidine using microwave irradiation (Scheme 90).142 Without using a base, no products were observed even after prolonged microwave irradiation. No pyridine product was observed, which confirmed that the reaction initiated with Michael addition of carbanion of 273 to nitroalkene 1, followed by cyclization and aromatization under air oxidation.
Recently, Ballini et al. demonstrated that reaction of β-nitroacrylates 275 with ylidene malononitriles 269 in Et3N/MeCN also afforded the penta-substituted anilines 276 with the presence of a cyano group in the o-position, an ester functionality in the m-position and an alkyl group in the o-position (Scheme 91).143 This procedure covers the difficulty of the previous works which need monosubstituted nitroalkenes for completion. Other catalytic systems such as KF/Al2O3, Amberlyst A 21, TMG, TMG/SiO2, DBU and CTAOH (cetyltrimethylammonium hydroxide) were also examined in this protocol with lower yields.
 | ||
| Scheme 91 Synthesis of penta-substituted anilines from β-nitroacrylates with ylidene malononitriles. | ||
Furthermore, reaction of 3-nitro-2H-chromenes 277 with 269 is investigated by Xie et al. in 2010 to give the corresponding fused benzene rings 278 (Scheme 92).144 They have shown that when R2 is hydrogen, the corresponding fused benzene rings can be obtained directly in high yields. But, with bulkier nucleophiles (R2 = CH3), the reaction gave the compounds 279 as products, which can be simply converted to the corresponding benzene derivatives 278 via treatment with NaOAc in refluxing ethanol.
When chromene 280 were used in the reaction with malononitrile derivatives 281 in the presence of Et3N, the corresponding CF3-containing functionalized 6H-dibenzo[b,d]pyrans 282 can be achieved in good to high yields (Scheme 93).145 It is notable that the 3-nitro-2-(trichloromethyl)-2H-chromenes do not lead to any desired product. In the case of 2-(1-phenylpropylidene)malononitriles 281 (R2 = CH3), the reaction proceeded in two steps with the formation of 7-amino-10-methyl-10-nitro-9-phenyl-6-(trifluoromethyl)-10,10a-dihydro-6H-benzo[c]chromene-8-carbonitriles (284), which were isolated as a result of a rare [1,5]-sigmatropic shift of the nitro group. Subsequent heating of this intermediate in the presence of NaOAc afforded the corresponding products 282. While the intermediates 284 are stable in solid state at room temperature for a long time, in DMSO-d6 solution they underwent reversible and stereospecific conversion into the corresponding isomers 283.
A regioselective one-pot synthesis of highly substituted 4-nitrophenols 287 is reported by Langer et al. in 2009 via [3 + 3] cyclizations of 1,3-bis(trimethylsilyloxy)-1,3-butadienes 285 with 3-ethoxy-2-nitro-2-en-1-ones 286 (Scheme 94).146 The best yield was obtained when 1.1 equiv. TiCl4 was used as catalyst in a highly concentrated solution of the reactants in CH2Cl2 at the temperature ranging from −78 to 20 °C. The proposed mechanism shows that the products are generated by removing the trimethylsilyl group from 285 and ethoxy group form 286 as TMSOEt. Subsequent reduction of the products in the presence of Pd/C gave corresponding substituted 4-aminophenols 288 in excellent yields.
Finally, 7-phenylindolo[3,2-a]carbazoles 289 were synthesized by Cachet group from readily available starting materials such as indoles 211 and β-nitrostyrenes 1 (Scheme 95).147 The authors proposed that the reaction proceeded via a sequential formation of indole dimer, conjugate addition of the resulting dimer to β-nitrostyrene and formal intramolecular [4 + 2]-cycloaddition. Different reaction conditions were examined and the use of an equimolar amount of SnCl2·H2O, MnO2, nitroalkene, and indole in anhydrous CH2Cl2 at room temperature under air atmosphere was selected as optimum conditions to give the corresponding products in moderate to good yields (20–66%). The reaction is not plausible with indoles with electron-withdrawing groups. Also, no result was achieved using anhydrous SnCl2 as catalyst.
9. Synthesis of naphthalene derivatives
Naphthalenes have been found extensively in the structure of biologically active natural products.148 For example, michellamines A–C have anti-HIV activities,149 korupensamines A–D have antimalarial activities,150 and (S)-gossypol has antifertility, anti-HIV, and anti-cancer activities.151 Also the naphthalene unit is a ubiquitous skeleton in optical and electronic materials.152 In addition, biaryl naphthalenes have found wide applications as chiral reagents in asymmetric synthesis.153 The first and most frequently used chiral phosphine ligand is BINAP.Several methods were developed for construction of naphthalene derivatives.154 Major synthetic strategies include the following: (1) Diels–Alder reactions,155 (2) transition metal-mediated annulations reactions,156 (3) transition metal-mediated electrocyclization,157 (4) ruthenium-catalysed ring-closing metathesis,158 (5) rearrangement of strained rings,159 (6) acid- and Lewis acid-catalysed intramolecular cyclizations,160 (7) phosphorus ylides in the synthesis of naphthalenes,161 (8) photochemically-mediated reactions,162 and (9) thermal cyclization reactions.163 However, these methods have certain drawbacks such as using relatively expensive catalysts, harsh reaction conditions, multi-step synthesis, and preparation of a mixture of isomers.
Xu et al. described the synthesis of polysubstituted naphthalenes 291 by the reaction of 2-(2-oxoethyl) benzaldehyde 290, as an analogue of pentane-1,5-dial, and nitroalkenes 1 (Scheme 96).164 Reaction was carried out using pyrrolidine as a catalyst and DMAP (0.2 equiv.) as an additive in methylene chloride in the presence of water to afford 3-nitro-2-aryl-1-naphthaldehyde 291 with moderate yield. The one-pot reaction consists of four consecutive reactions that include a cascade Michael–Henry reaction, a dehydration reaction, and an aromatization reaction via oxidation in air. This reaction is only possible for nitroolefins derived from aromatic aldehydes and aliphatic nitroalkenes did not give the corresponding naphthalenes.
Very recently, an interesting approach for synthesis of 2-aminonaphthalenes from their nitro analogue 295 is presented by Kürti and keene (Scheme 97).165 They described that addition of a nitroalkene 38 to a solution of lithiated o-tolualdehyde tert-butyl imine 293, which was prepared from o-tolualdehyde tert-butyl imine 292 and a stoichiometric amount of n-butyllithium in the presence of catalytic amounts (15 mol%) of 2,2,6,6-tetramethylpiperidine (TMP) in THF at 0 °C, afforded a novel cyclic nitrostyrenes 294. These compounds can be simply aromatized to 3-substituted-2-nitronaphthalenes 295 via treatment with N-bromosuccinimide in the presence of benzoyl peroxide. Aromatization of 294 via dehydrogenation with Pd/C, DDQ, and refluxing in high-boiling solvents was unsuccessful. In addition, the authors have shown that this protocol is suitable for large scale synthesis of substituted 2-aminonaphthalenes via reduction of the nitro group.
10. Conclusions
Conjugated nitroalkenes take part in a wide variety of reactions such as Michael addition, Diels–Alder reaction, 1,3-dipolar and cycloaddition, Morita–Baylis–Hillman reaction and many cascade reactions to provide novel carbocycles often with high regio- and stereoselectivities. Such nitroalkenes could be cyclic or acyclic and possess a variety of substituents, viz electroneutral, electron donating and electron withdrawing, at α- and/or β-positions. These substrates have the adaptability to a variety of reaction conditions, including metal mediated and organocatalytic, owing to the co-ordinating ability of nitro group. This versatile reactivity profile of nitroalkenes has transformed them into fabulous substrates in the synthesis of small, common and medium ring carbocycles, including natural products. These developments testify that synthetic organic chemists have traversed from anera of ‘nitrophobia’ to one of ‘nitromania’.Abbreviations
| AcOH | Acetic acid |
| Ar | Aryl |
| Binap | 2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl |
| Bu | Butyl |
| Bn | Benzyl |
| Bz | Benzoyl |
| CTAOH | Cetyltrimethylamonium hydroxide |
| DA | Diels–Alder |
| DABCO | 1,4-Diazabicyclo[2.2.2]octane |
| dba | Dibenzylideneacetone |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| DCE | Dichloroethane |
| DCM | Dichloromethane |
| DDQ | 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone |
| de | Diastereomeric excess |
| DMAP | 4-Dimethylaminopyridine |
| DMF | Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| dr | Diastereomeric ratio |
| ee | Enantiomeric excess |
| Et | Ethyl |
| HOMO | Highest occupied molecular orbital |
| IBX | 2-Iodoxybenzoic acid |
| IMDA | Intramolecular Diels–Alder |
| Mes | Mesityl |
| MW | Microwave |
| 4-NBA | 4-Nitrobenzoic acid |
| NBS | N-Bromosuccinimide |
| NEDDA | Normal-electron-demand Diels–Alder |
| OFBA | o-Fluorobenzoic acid |
| PCC | Pyridinium chlorochromate |
| Ph | Phenyl |
| PPTS | Pyridinium p-toluenesulfonate |
| PTSA | p-Toluenesulfonic acid |
| SDBS | Sodium dodecylbenzenesulfonate |
| TBAF | Tetrabutylammonium fluoride |
| t-BuO | tert-Butoxide |
| TEA | Teriethylamine |
| TES | Triethylsilyl |
| Tf | Trifluoromethane sulfonyl |
| TFA | Trifluoroacetic acid |
| THF | Tetrahydrofuran |
| TLC | Thin-layer chromatography |
| TMG | Tetramethylguanidine |
| TMM | Trimethylene methane |
| TMS | Trimethylsilyl |
Acknowledgements
Azim Ziyaei Halimehjani and Seyyed Emad Hooshmand thank the Faculty of Chemistry of Kharazmi University for supporting their research. Also, INNN thanks Department of Science and Technology (DST), India, for supporting research in the area of nitroalkene chemistry.References
- General reviews/books: (a) A. G. M. Barrett and G. G. Graboski, Chem. Rev., 1986, 86, 751 CrossRef CAS; (b) Nitroalkanes and Nitroalkenes in Synthesis: Tetrahedron Symposia-in-Print, No. 41: [in: Tetrahedron, 1990; 46(21)], ed. A. G. M. Barrett, Pergamon, Oxford, UK, 1990, p. 285 Search PubMed; (c) V. V. Perekalin, E. S. Lipina, V. M. Berestovitskaya and D. A. Efremov, Nitroalkenes: Conjugated Nitro Compounds, Wiley, Chichester, UK, 1994, p. 256 Search PubMed; (d) R. Ballini, S. Gabrielli and A. Palmieri, Green Chemistry for Environmental Sustainability, 2011, p. 53 Search PubMed; (e) R. Ballini, S. Gabrielli and A. Palmieri, Curr. Org. Chem., 2010, 14, 65 CrossRef CAS; (f) A. G. M. Barrett, Chem. Soc. Rev., 1991, 20, 95 RSC; (g) K. Banert, Sci. Synth., 2006, 747 Search PubMed.
- Nitroalkenes as Michael acceptors, reviews/books: (a) O. M. Berner, L. Tedeschi and D. Enders, Eur. J. Org. Chem., 2002, 12, 1877 CrossRef; (b) Anon, Chemtracts, 2010, 23, 58 Search PubMed; (c) D. M. Barnes, Asymmetric Catalysis on Industrial Scale, ed. H.-U. Blaser and H.-J. Federsel, Wiley-VCH Verlag GmbH, 2nd edn, 2010, p. 439 Search PubMed; (d) D. Roca-Lopez, D. Sadaba, I. Delso, R. P. Herrera, T. Tejero and P. Merino, Tetrahedron: Asymmetry, 2010, 21, 2561 CrossRef CAS; (e) R. Somanathan, D. Chavez, F. A. Servin, J. A. Romero, A. Navarrete, M. Parra-Hake, G. Aguirre, C. Anaya de Parrodi and J. Gonzalez, Curr. Org. Chem., 2012, 16, 2440 CrossRef CAS.
- Nitroalkenes as cycloaddition partners, reviews: (a) S. E. Denmark and A. Thorarensen, Chem. Rev., 1996, 96, 137 CrossRef CAS PubMed; (b) G. K. Trivedi, J. Indian Chem. Soc., 2004, 81, 187 CAS; (c) N. Ono, Heterocycles, 2008, 75, 243 CrossRef CAS.
- Morita–Baylis–Hillman reaction of nitroalkenes, review: (a) K. Kaur and I. N. N. Namboothiri, Chimia, 2012, 66, 913 CrossRef CAS PubMed.
- Nitroalkenes in the synthesis of heterocycles, reviews: (a) R. S. Varma and G. W. Kabalka, Heterocycles, 1986, 24, 2645 CrossRef; (b) W. Yan, X. Shi and C. Zhong, Asian J. Org. Chem., 2013, 2, 904 CrossRef CAS.
- Nitro group manipulation, reviews/books: (a) G. W. Kabalka and R. S. Varma, Org. Prep. Proced. Int., 1987, 19, 283 CrossRef CAS; (b) R. Ballini, E. Marcantoni and M. Petrini, Amino Group Chemistry, ed. A. Ricci, 2008, p. 93 Search PubMed.
- Natural product synthesis, reviews: S. V. Ley and C. E. Gutteridge, Chemtracts: Org. Chem., 1995, 8, 222 CAS; S. V. Ley and C. E. Gutteridge, Chemtracts: Inorg. Chem., 1996, 8, 166 Search PubMed.
- Henry reaction, reviews/books: (a) A. Hassner and I. N. N. Namboothiri, Organic Syntheses Based on Named Reactions, Elsevier, Oxford, UK, 3rd edn, A Practical Guide, 2012 Search PubMed; (b) A. X. Wang, Name Reactions for Homologations, ed. J. J. Li, John Wiley & Sons, 2009, p. 404 Search PubMed.
- (a) S. Maity, T. Naveen, U. Sharma and D. Maiti, Org. Lett., 2013, 15, 3384 CrossRef CAS PubMed; (b) H. M. Chawla and R. S. Mittal, Synthesis, 1985, 70 CrossRef CAS; (c) R. Ballini, R. Castagnani and M. Petrini, J. Org. Chem., 1992, 57, 2160 CrossRef CAS; (d) S. S. Jew, H. D. Kim, Y. S. Cho and C. H. Cook, Chem. Lett., 1986, 1747 CrossRef CAS; (e) M. Ganesh and I. N. N. Namboothiri, Tetrahedron, 2007, 63, 11973 CrossRef CAS.
- Nitroalkyl group as 1,3-dipole precursor: (a) I. N. N. Namboothiri and N. Rastogi, Top. Heterocycl. Chem., Synthesis of Heterocycles via Cycloadditions I, ed. A. Hassner, Springer-Verlag, Germany, 2008, vol. 12, p. 1 Search PubMed; (b) I. N. N. Namboothiri and A. Hassner, Top. Curr. Chem., Stereoselective Heterocyclic Synthesis III, ed. P. Metz, Springer-Verlag, Germany, 2001, vol. 216, p. 1 Search PubMed.
- (a) J. Salaun, Top. Curr. Chem., 2000, 207, 1 CrossRef CAS; (b) R. Faust, Angew. Chem., Int. Ed., 2001, 40, 2251 CrossRef CAS; (c) F. Gnad and O. Reiser, Chem. Rev., 2003, 103, 1603 CrossRef CAS.
- H. W. Liu and C. T. Walsh, The Chemistry of the Cyclopropyl Group, ed. Z. Rappoport, John Wiley, New York, NY, 1997, p. 959 Search PubMed.
- H. Pellissier, Tetrahedron, 2012, 68, 2197 CrossRef CAS and references are therein.
- Y.-N. Xuan, S.-Z. Nie, L.-T. Dong, J.-M. Zhang and M. Yan, Org. Lett., 2009, 11, 1583 CrossRef CAS PubMed.
- A. Russo and A. Lattanzi, Tetrahedron: Asymmetry, 2010, 21, 1155 CrossRef CAS.
- H. J. Lee, S. M. Kim and D. Y. Kim, Tetrahedron Lett., 2012, 53, 3437 CrossRef CAS.
- R. Fan, Y. Ye, W. Li and L. Wang, Adv. Synth. Catal., 2008, 350, 2488 CrossRef CAS.
- A. Y. Barkov, V. Y. Korotaev and V. Y. Sosnovskikh, Tetrahedron Lett., 2013, 54, 4181 CrossRef CAS.
- (a) N. Gauvry, C. Lescop and F. Huet, Eur. J. Org. Chem., 2006, 5207 CrossRef CAS; (b) F. Toda and P. Garratt, Chem. Rev., 1992, 92, 1685 CrossRef CAS; (c) A. K. Mailyan, A. S. Peregudov, P. H. Dixneuf, C. Bruneau and S. N. Osipov, J. Org. Chem., 2012, 77, 8518 CrossRef CAS PubMed; (d) M. Mori, H. Wakamatsu, K. Tonogaki, R. Fujita, T. Kitamura and Y. Sato, J. Org. Chem., 2005, 70, 1066 CrossRef CAS PubMed.
- (a) W. A. Kinney, M. Abou-Gharbia, D. T. Garrison, J. Schmid, D. M. Kowal, D. R. Bramlett, T. L. Miller, R. P. Tasse, M. M. Zaleska and J. A. Moyer, J. Med. Chem., 1998, 41, 236 CrossRef CAS PubMed; (b) P. C. M. Chan, R. J. Roon, J. F. Koerner, N. J. Taylor and J. F. Honek, J. Med. Chem., 1995, 38, 4433 CrossRef CAS PubMed; (c) G. Lai, J. R. Merritt, Z. He, D. Feng, J. Chao, M. F. Czarniecki, L. L. Rokosz, T. M. Stauffer, D. Rindgen and R. G. Taveras, Bioorg. Med. Chem. Lett., 2008, 18, 1864 CrossRef CAS PubMed; (d) R. Alibes, P. de March, M. Figueredo, J. Font, M. Racamonde and T. Parella, Org. Lett., 2004, 6, 1449 CrossRef CAS PubMed.
- D. L. Smith, S. R. Chidipudi, W. R. Goundry and H. W. Lam, Org. Lett., 2012, 14, 4934 CrossRef CAS PubMed.
- For an example of a similar oxidative cyclization involving an alkyne, an alkene, and Ni(0), see: H. P. Hratchian, S. K. Chowdhury, V. M. Gutierrez-Garcia, K. K. D. Amarasinghe, M. J. Heeg, H. B. Schlegel and J. Montgomery, Organometallics, 2004, 23, 4636 CrossRef CAS.
- V. Y. Korotaev, A. Y. Barkov, P. A. Slepukhin, M. I. Kodess and V. Y. Sosnovskikh, Tetrahedron Lett., 2011, 52, 5764 CrossRef CAS.
- G. Talavera, E. Reyes, J. L. Vicario and L. Carrillo, Angew. Chem., Int. Ed., 2012, 51, 4104 CrossRef CAS PubMed.
- For reviews on the synthesis and applications of cyclopentanes, see: (a) L. F. Silva, Tetrahedron, 2002, 58, 9137 CrossRef CAS; (b) R. J. Ferrier and S. Middleton, Chem. Rev., 1993, 93, 2779 CrossRef CAS; (c) B. M. Trost, Angew. Chem., Int. Ed., 1986, 25, 1 CrossRef.
- For reviews: (a) H. Pellissier, Tetrahedron, 2007, 63, 3235 CrossRef CAS; (b) G. Pandey, P. Banerjee and S. R. Gadre, Chem. Rev., 2006, 106, 4484 CrossRef CAS PubMed.
- (a) W. S. Jen, J. J. Wiener and D. W. C. MacMillan, J. Am. Chem. Soc., 2000, 122, 9874 CrossRef CAS; (b) For recent reviews on phosphine-catalyzed [3 + 2] cycloadditions, see: L.-W. Ye, J. Zhou and Y. Tang, Chem. Soc. Rev., 2008, 37, 1140 RSC; (c) B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102 RSC.
- (a) J. C. Wasilke, S. J. Obrey, R. T. Baker and G. C. Bazan, Chem. Rev., 2005, 105, 1001 CrossRef CAS; (b) J. Zhu and H. Bienayme, Multicomponent Reactions, Wiley-VCH, Weinheim, 2005 Search PubMed; (c) L. F. Tietze, G. Brasche and K. Gericke, Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2006 Search PubMed; (d) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134 CrossRef CAS PubMed; (e) H.-C. Guo and J.-A. Ma, Angew. Chem., Int. Ed., 2006, 45, 354 CrossRef CAS PubMed.
- (a) For books, see: A. Berkessel and H. Groger, Asymmetric Organocatalysis – From Biomimetic Concepts to Powerful Methods for Asymmetric Synthesis, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) P. I. Dalko, Enantioselective Organocatalysis Reactions and Experimental Procedure, Wiley-VCH, Weinheim, 2007 Search PubMed.
- B.-C. Hong, R. Y. Nimje, C.-W. Lin and J.-H. Liao, Org. Lett., 2011, 13, 1278 CrossRef CAS PubMed.
- (a) S. Li, Nat. Prod. Rep., 2010, 27, 57 RSC; (b) K. A. Miller, S. Tsukamoto and R. M. Williams, Nat. Chem., 2009, 1, 63 CrossRef CAS PubMed; (c) T. J. Greshock, A. W. Grubbs, P. Jiao, D. T. Wicklow, J. B. Gloer and R. M. Williams, Angew. Chem., Int. Ed., 2008, 47, 3573 CrossRef CAS PubMed; (d) K. A. Millera and R. M. Williams, Chem. Soc. Rev., 2009, 38, 3160 RSC.
- A. Fensome, W. R. Adams, A. L. Adams, T. J. Berrodin, J. Cohen, C. Huselton, A. Illenberger, J. C. Kern, V. A. Hudak, M. A. Marella, E. G. Melenski, C. C. McComas, C. A. Mugford, O. D. Slayden, M. Yudt, Z. Zhang, P. Zhang, Y. Zhu, R. C. Winneker and J. E. Wrobel, J. Med. Chem., 2008, 51, 1861 CrossRef CAS PubMed.
- X. Li, Y.-M. Li, F.-Z. Peng, S.-T. Wu, Z.-Q. Li, Z.-W. Sun, H.-B. Zhang and Z.-H. Shao, Org. Lett., 2011, 13, 6160 CrossRef CAS.
- Y.-M. Li, X. Li, F.-Z. Peng, Z.-Q. Li, S.-T. Wu, Z.-W. Sun, H.-B. Zhang and Z.-H. Shao, Org. Lett., 2011, 13, 6200 CrossRef CAS PubMed.
- W. Raimondi, G. Lettieri, J.-P. Dulcere, D. Bonne and J. Rodriguez, Chem. Commun., 2010, 7247 RSC.
- B. Tan, P. J. Chua, X. Zeng, M. Lu and G. Zhong, Org. Lett., 2008, 10, 3489 CrossRef CAS PubMed.
- B. Tan, Z. Shi, P. J. Chua and G. Zhong, Org. Lett., 2008, 10, 3425 CrossRef CAS PubMed.
- Q. An, J. Shen, N. Butt, D. Liu, Y. Liu and W. Zhang, Synthesis, 2013, 1612 CAS.
- M. Tsakos, M. R. J. Elsegood and C. G. Kokotos, Chem. Commun., 2013, 2219 RSC.
- B. M. Trost, D. A. Bringley and P. S. Seng, Org. Lett., 2012, 14, 234 CrossRef CAS PubMed.
- B. M. Trost, D. A. Bringley and B. M. O'Keefe, Org. Lett., 2013, 15, 5630 CrossRef CAS PubMed.
- M. Guillaume, E. Dumez, J. Rodriguez and J.-P. Duclere, Synlett, 2002, 1883 CAS.
- G. R. Boyce and J. S. Johnson, Angew. Chem., Int. Ed., 2010, 49, 8930 CrossRef CAS PubMed.
- F. Felluga, C. Forzato, P. Nitti, G. Pitacco, F. Prati, E. Valentin and E. Zangrando, Chirality, 2012, 24, 1005 CrossRef CAS PubMed.
- D. Ding, C.-G. Zhao, Q. Guo and H. Arman, Tetrahedron, 2010, 66, 4423 CrossRef CAS.
- A. Sagirli, Y. Durust, B. Kariuki and D. W. Knight, Tetrahedron, 2013, 69, 69 CrossRef.
- B.-C. Hong, N. S. Dange, C.-S. Hsu and J. H. Liao, Org. Lett., 2010, 12, 4812 CrossRef CAS PubMed.
- X.-Y. Guan, Y. Wei and M. Shi, Org. Lett., 2010, 12, 5024 CrossRef CAS PubMed.
- C. E. Henry and O. Kwon, Org. Lett., 2007, 9, 3069 CrossRef CAS.
- A. J. Clarke and D. A. Hunt, Tetrahedron Lett., 2009, 50, 2949 CrossRef CAS.
- G. R. Boyce, S. Liu and J. S. Johnson, Org. Lett., 2012, 14, 652 CrossRef CAS PubMed.
- P. Shanbhag, P. R. Nareddy, M. Dadwal, S. M. Mobin and I. N. N. Namboothiri, Org. Biomol. Chem., 2010, 8, 4867 CAS.
- (a) T. Ishikawa, K. Kudo, K. Kuroyabu, S. Uchida, T. Kudoh and S. Saito, J. Org. Chem., 2008, 73, 7498 CrossRef CAS PubMed; (b) H. Hilpert and B. Wirz, Tetrahedron, 2001, 57, 681 CrossRef CAS; (c) J. L. Bicas, A. P. Dioniísio and G. M. Pastore, Chem. Rev., 2009, 109, 4518 CrossRef CAS PubMed; (d) E. Bellavance, V. Luu-The and D. Poirier, J. Med. Chem., 2009, 52, 7488 CrossRef CAS PubMed; (e) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS PubMed; (f) X. Zhang, C. Hou, H. Hufnagel, M. Singer, E. Opas, S. McKenney, D. Johnson and Z. Sui, ACS Med. Chem. Lett., 2012, 3, 1039 CrossRef CAS PubMed.
- (a) N. Kazuoki, M. Kamoshita, D. Takayuki, H. Yamada and T. Takahashi, Tetrahedron Lett., 2001, 42, 7855 CrossRef; (b) W. Zhang, R. Benmohamed, A. C. Arvanites, R. I. Morimoto, R. J. Ferrante, D. R. Kirsch and R. B. Silverman, Bioorg. Med. Chem., 2012, 20, 1029 CrossRef CAS PubMed; (c) Y. Zhang, R. Benmohamed, W. Zhang, J. Kim, C. K. Edgerly, Y. Zhu, R. I. Morimoto, R. J. Ferrante, D. R. Kirsch and R. B. Silverman, ACS Med. Chem. Lett., 2012, 3, 584 CrossRef CAS; (d) S. Pogny, S. Nouri, A. Chiaroni, M. Guyot and N. Samadi, J. Org. Chem., 2001, 66, 7263 CrossRef; (e) S. Jawamatsi, K. Matsubara and H. Nagashima, J. Org. Chem., 1999, 64, 9625 CrossRef; (f) E. Arce, M. C. Carreno, M. B. Cid and J. L. Ruano, J. Org. Chem., 1994, 59, 3421 CrossRef CAS; (g) M. Node, H. Imazato, R. Kurosaki, Y. Kawano, T. Inoue, K. Nishide and K. Fuji, Heterocycles, 1996, 42, 811 CrossRef CAS; (h) V. Cere, G. Montovani, F. Peri, S. Pollicino and A. A. Ricci, Tetrahedron, 2000, 56, 1225 CrossRef CAS.
- T. Yasuhara, K. Nishimura, M. Yamashita, N. Fukuyama, K. Yamada, O. Muraoka and K. Tomioka, Org. Lett., 2003, 5, 1123 CrossRef CAS PubMed.
- S. Rajkumar, K. Shankland, G. D. Brown and A. J. A. Cobb, Chem. Sci., 2012, 3, 584 RSC.
- B. Tan, P. J. Chua, Y. Li and G. Zhong, Org. Lett., 2008, 10, 2437 CrossRef CAS PubMed.
- Y. Hayashi, T. Okano, S. Aratake and D. Hazelard, Angew. Chem., Int. Ed., 2007, 46, 4922 CrossRef CAS PubMed.
- C. T. Wong, Tetrahedron, 2012, 68, 481 CrossRef CAS.
- P. Chintala, S. K. Ghost, F. Long, A. D. Headley and B. Ni, Adv. Synth. Catal., 2011, 353, 2905 CrossRef CAS.
- B. C. Hong, D. J. Lan, N. S. Dange, G. H. Lee and J. H. Liao, Eur. J. Org. Chem., 2013, 2472 CrossRef CAS.
- B. C. Hong, P. Kotame and J. H. Liao, Org. Biomol. Chem., 2011, 9, 382 CAS.
- Y. Wang, W. Yuan, H.-F. Zheng and D.-Q. Shi, Synthesis, 2013, 382 Search PubMed.
- (a) O. Basle, W. Raimondi, M. del Mar Sanchez Duque, D. Bonne, T. Constantieux and J. Rodriguez, Org. Lett., 2010, 12, 5246 CrossRef CAS PubMed; (b) W. Raimondi, M. M. S. Duque, S. Goudedranche, A. Quintard, T. Constantieux, X. Bugaut, D. Bonne and J. Rodriguez, Synthesis, 2013, 1659 CAS.
- D. Enders, G. Urbanietz, E. Cassens-Sasse, S. Keeb and G. Raabe, Adv. Synth. Catal., 2012, 354, 1481 CrossRef CAS.
- Z. Mao, Y. Jia, Z. Xu and R. Wang, Adv. Synth. Catal., 2012, 354, 1401 CrossRef CAS.
- S. Varga, G. Jakab, L. Drahos, T. Holczbauer, M. Czugler and T. Soos, Org. Lett., 2011, 13, 5416 CrossRef CAS PubMed.
- Y. Wang, R.-G. Han, Y.-L. Zhao, S. Yang, P.-F. Xu and D. J. Dixon, Angew. Chem., Int. Ed., 2009, 48, 9834 CrossRef CAS.
- S. Roy, M. Amireddy and K. Chen, Tetrahedron, 2013, 69, 8751 CrossRef CAS.
- D. Enders, R. Hahn and I. Atodiresei, Adv. Synth. Catal., 2013, 355, 1126 CrossRef CAS.
- D. F. Yu, Y. Wang and P. F. Xu, Adv. Synth. Catal., 2011, 353, 2960 CrossRef CAS.
- (a) W. Zou, K. Vembaiyan, M. Bhasin and D. T. Williams, Carbohydr. Res., 2009, 344, 2144 CrossRef CAS PubMed; (b) W. Zou, M. Bhasin, K. Vembaiyan and D. T. Williams, Carbohydr. Res., 2009, 344, 1024 CrossRef CAS PubMed.
- M. Crawshaw, N. W. Hird, K. Irie and K. Naga, Tetrahedron Lett., 1997, 38, 7115 CrossRef CAS.
- N. W. Hird, K. Irie and K. Nagai, Tetrahedron Lett., 1997, 38, 7111 CrossRef CAS.
- P. He, X. Liu, J. Shi, L. Lin and X. Feng, Org. Lett., 2011, 13, 936 CrossRef CAS PubMed.
- Y. Hoashi, T. Yabuta, P. Yuan, H. Miyabe and Y. Takemoto, Tetrahedron, 2006, 62, 365 CrossRef CAS.
- N. Ayyagari, D. Jose, S. M. Mobin and I. N. N. Namboothiri, Tetrahedron Lett., 2011, 52, 258 CrossRef CAS.
- N. Ayyagari and I. N. N. Namboothiri, Tetrahedron: Asymmetry, 2012, 23, 605 CrossRef CAS.
- L. Y. Wu, G. Bencivenni, M. Mancinelli, A. Mazzanti, G. Bartoli and P. Melchiorre, Angew. Chem., Int. Ed., 2009, 48, 7196 CrossRef CAS PubMed.
- R. Thayumanavan, B. Dhevalapally, K. Sakthivel, F. Tanaka and C. F. Barbas III, Tetrahedron Lett., 2002, 43, 3817 CrossRef CAS.
- C. L. Cao, Y. Y. Zhou, J. Zhou, X. L. Sun, Y. Tang, Y. X. Li, G. Y. Li and J. Sun, Chem.–Eur. J., 2009, 15, 11384 CrossRef CAS PubMed.
- F. Cagide-Fajín, O. Nieto-García, H. Lago-Santomé and R. Alonso, J. Org. Chem., 2012, 77, 11377 CrossRef PubMed.
- G. J. Kuster and H. W. Scheeren, Tetrahedron Lett., 2000, 41, 515 CrossRef CAS.
- M. M. Sarmah, D. Prajapati and W. Hu, Synlett, 2013, 471 CAS.
- (a) S. E. Denmark and M. Seierstad, J. Org. Chem., 1999, 64, 1610 CrossRef CAS; (b) S. E. Denmark and R. Y. Baiazitov, Org. Lett., 2005, 7, 5617 CrossRef CAS PubMed.
- (a) M. J. Kurth, M. J. O'Brien, H. Hope and M. Yanuck, J. Org. Chem., 1985, 50, 2626 CrossRef CAS; (b) C. Retherford and P. Knochel, Tetrahedron Lett., 1991, 32, 441 CrossRef CAS; (c) L. Sader-Bakaouni, O. Charton, N. Kunesch and F. Tillequin, Tetrahedron, 1998, 54, 1773 CrossRef CAS; (d) D. R. Williams and T. A. Brugel, Org. Lett., 2000, 2, 1023 CrossRef CAS PubMed.
- A. Aguado and N. Takenaka, Synlett, 2011, 1259 CAS.
- P. A. Wade, J. K. Murray, A. Pipic, R. J. Arbaugh and A. Jeyarajasingam, J. Phys. Org. Chem., 2009, 22, 337 CrossRef CAS.
- V. A. Zapol'skii, J. C. Namyslo, B. Blaschkowski and D. E. Kaufmann, Synlett, 2006, 3464 Search PubMed.
- (a) Z.-J. Jia, H. Jiang, J.-L. Li, B. Gschwend, Q.-Z. Li, X. Yin, J. Grouleff, Y.-C. Chen and K. A. Jørgensen, J. Am. Chem. Soc., 2011, 133, 5053 CrossRef CAS; (b) Z.-J. Jia, Q. Zhou, Q.-Q. Zhou, P.-Q. Chen and Y.-C. Chen, Angew. Chem., Int. Ed., 2011, 50, 8638 CrossRef CAS PubMed.
- Y. Liu, M. Nappi, E. Arceo, S. Vera and P. Melchiorre, J. Am. Chem. Soc., 2011, 133, 15212 CrossRef CAS.
- X.-F. Wang, J.-R. Chen, Y.-J. Cao, H.-G. Cheng and W.-J. Xiao, Org. Lett., 2010, 12, 1140 CrossRef CAS PubMed.
- D. Enders, R. Krull and W. Bettray, Synthesis, 2010, 567 CrossRef CAS.
- D. Enders, C. Wang, M. Mukanova and A. Greb, Chem. Commun., 2010, 46, 2447 RSC.
- N. Erdmann, A. R. Philipps, L. Atodiresei and D. Enders, Adv. Synth. Catal., 2013, 355, 847 CrossRef CAS.
- F.-L. Zhang, A.-W. Xu, Y.-F. Gong, M.-H. Wei and X.-L. Yang, Chem.–Eur. J., 2009, 15, 6815 CrossRef CAS.
- D. Enders, M. R. M. Huettl, C. Grondal and G. Raabe, Nature, 2006, 441, 861 CrossRef CAS.
- D. Enders, M. R. M. Huttl, J. Runsink, G. Raabe and B. Wendt, Angew. Chem., Int. Ed., 2007, 46, 467 CrossRef CAS.
- N. Erdmann, I. Atodiresei and D. Enders, Synthesis, 2012, 2107 Search PubMed; D. Enders, B. Schmid, N. Erdmann and G. Raabe, Synthesis, 2010, 2271 CrossRef CAS.
- Y. Jia, Z. Mao and R. Wang, Tetrahedron: Asymmetry, 2011, 22, 2018 CrossRef CAS.
- M. Rueping, K. L. Haack, W. Ieawsuwan, H. Sundén, M. Blanco and F. R. Schoepke, Chem. Commun., 2011, 47, 3828 RSC.
- X. Zeng, Q. Ni, G. Raabe and D. Enders, Angew. Chem., Int. Ed., 2013, 52, 2997 Search PubMed.
- B.-C. Hong, R.-H. Jan, C.-W. Tsai, R. Y. Nimje, J.-H. Liao and G.-H. Lee, Org. Lett., 2009, 11, 5246 CrossRef CAS.
- B. Tan, D. Zhu, L. Zhang, P. J. Chua, X. Zeng and G. Zhong, Chem.–Eur. J., 2010, 16, 3842 CrossRef CAS.
- (a) P. Kotame, B. C. Hong and J. H. Liao, Tetrahedron Lett., 2009, 50, 704 CrossRef CAS; (b) B. C. Hong, P. Kotame, C. W. Tsai and J. H. Liao, Org. Lett., 2010, 12, 776 CrossRef CAS.
- (a) H. Ishikawa, T. Suzuki, H. Orita, T. Uchimaru and Y. Hayashi, Chem.–Eur. J., 2010, 16, 12616 CrossRef CAS; (b) H. Ishikawa, T. Suzuki and Y. Hayashi, Angew. Chem., Int. Ed., 2009, 48, 1304 CrossRef CAS.
- E. R. Barnum and C. S. Hamilton, J. Am. Chem. Soc., 1942, 64, 540 CrossRef CAS.
- S. Zhu, S. Yu, Y. Wang and D. Ma, Angew. Chem., Int. Ed., 2010, 49, 4656 CrossRef CAS.
- A. Krowczynski and L. Kozerski, Synthesis, 1983, 489 CrossRef CAS.
- Naturally Occurring Phorbol Esters, ed. F. J. Evans, CRC, Boca Raton, F. L, 1986 Search PubMed.
- S. M. Bondi and J. Clardy, J. Am. Chem. Soc., 2001, 123, 9900 CrossRef.
- H.-F. Wong and G. D. Brown, J. Nat. Prod., 2002, 65, 481 CrossRef CAS.
- A. D. Patil, L. Killmer, P. Offen, B. Carte, A. J. Jurewicz and R. K. Johnson, Tetrahedron, 1997, 53, 5047 CrossRef CAS.
- (a) M. A. Battiste, P. M. Pelphrey and D. L. Wright, Chem.–Eur. J., 2006, 12, 3438 CrossRef CAS; (b) S. J. Rhoads and C. F. Brandenburg, J. Am. Chem. Soc., 1971, 93, 5805 CrossRef CAS; (c) T. Fujimoto, Y. Kodama, I. Yamamoto and A. Kakehi, J. Org. Chem., 1997, 62, 6627 CrossRef CAS; (d) Y. Ni and J. Montgomery, J. Am. Chem. Soc., 2004, 126, 11162 CrossRef CAS; (e) N. S. Dange, B.-C. Hong, C.-C. Lee and G.-H. Lee, Org. Lett., 2013, 15, 3914 CrossRef CAS; (f) T. V. Ovaska, J. A. Sullivan, S. I. Ovaska, J. B. Winegrad and J. D. Fair, Org. Lett., 2009, 11, 2715 CrossRef CAS; (g) L. F. Silva, J. R. S. Vasconcelos and M. A. Nogueira, Org. Lett., 2008, 10, 1017 CrossRef CAS; (h) S. Saito, S. Komagawa, I. Azumaya and M. Masuda, J. Org. Chem., 2007, 72, 9114 CrossRef CAS.
- S. Xu, Y. Zhou, J. Xu, H. Jiang and H. Liu, Green Chem., 2013, 15, 718 RSC.
- C. C. J. Loh, J. Badorrek, G. Raabe and D. Enders, Chem.–Eur. J., 2011, 17, 13409 CrossRef CAS.
- (a) R. A. Yoder and J. N. Johnston, Chem. Rev., 2005, 105, 4730 CrossRef CAS; (b) T. Tokoroyama, Synthesis, 2000, 611 CrossRef CAS; (c) A. T. Merritt and S. V. Ley, Nat. Prod. Rep., 1992, 9, 243 RSC; (d) J. R. Hanson, Nat. Prod. Rep., 2010, 27, 887 RSC; (e) M. Ibrahim-Ouali, Steroids, 2009, 74, 133 CrossRef CAS; (f) C. F. Nising and S. Bräse, Angew. Chem., Int. Ed., 2008, 47, 9389 CrossRef CAS; (g) M. Ibrahim-Ouali, Steroids, 2007, 72, 475 CrossRef CAS; (h) M. Ibrahim-Ouali and M. Santelli, Steroids, 2006, 71, 1025 CrossRef CAS; (i) T. J. Maimone and P. S. Baran, Nat. Chem. Biol., 2007, 3, 396 CrossRef CAS.
- (a) V. Singh, S. R. Iyer and S. Pal, Tetrahedron, 2005, 61, 9197 CrossRef CAS; (b) J. Mulzer, D. Castagnolo, W. Felzmann, S. Marchart, C. Pilger and V. S. Enev, Chem.–Eur. J., 2006, 12, 5992 CrossRef CAS; (c) C.-C. Liao, Pure Appl. Chem., 2005, 77, 1221 CAS; (d) M. A. Varner and R. B. Grossman, Tetrahedron, 1999, 55, 13867 CrossRef CAS; (e) E. Suzuki, M. Ueda, S. Ohba, T. Sugai and M. Shoji, Tetrahedron Lett., 2013, 54, 1589 CrossRef CAS.
- (a) F. F. Paintner, G. Bauschke and K. Polborn, Tetrahedron Lett., 2003, 44, 2549 CrossRef CAS; (b) S. C. Butler and C. J. Forsyth, J. Org. Chem., 2013, 78, 3895 CrossRef CAS; (c) L. Chen, Z. Hua, G. Li and Z. Jin, Org. Lett., 2011, 13, 3580 CrossRef CAS.
- D. R. Williams, J. C. Klein and N. S. C. Chow, Tetrahedron Lett., 2011, 52, 2120 CrossRef CAS.
- W. K. Chung and P. Chiu, Synlett, 2005, 55 CAS.
- (a) Functional Organic Materials, Synthesis, Strategies and Applications, ed. T. J. J. Müller and U. H. F. Bunz, Wiley-VCH, Weinheim, 2007 Search PubMed; (b) R. H. Thomson, The Chemistry of Natural Products, Blackie, Glasgow, 1985 Search PubMed; (c) F. V. Singh, R. Vatsyayan, U. Roy and A. Goel, Bioorg. Med. Chem. Lett., 2006, 16, 2734 CrossRef CAS; (d) N. C. Warshakoon, J. Sheville, R. T. Bhatt, W. Ji, J. L. Mendez-Andino, K. M. Meyers, N. Kim, J. A. Wos, C. Mitchell, J. L. Paris, B. B. Pinney, O. Reizes and X. E. Hu, Bioorg. Med. Chem. Lett., 2006, 16, 5207 CrossRef CAS; (e) J. Q. Wang, M. Gao, K. D. Miller, G. W. Sledge and Q.-H. Zheng, Bioorg. Med. Chem. Lett., 2006, 16, 4102 CrossRef CAS; (f) T. Kaiah, B. P. V. Lingaiah, B. Narsaiah, B. Shireesha, B. A. Kumar, S. Gururaj, T. Pathasarathy and B. Sridhar, Bioorg. Med. Chem. Lett., 2007, 17, 3445 CrossRef; (g) R. Romagnoli, P. G. Baraldi, M. D. Carrion, G. L. Cara, D. Preti, F. Fruttarolo, M. G. Parani, M. A. Tabrizi, M. Tolomeo, S. Grimando, A. D. Cristina, J. Balazarini, J. A. Hadfield, A. Brancale and E. Hamel, J. Med. Chem., 2007, 50, 2273 CrossRef CAS.
- (a) D. Maitraie, G. V. Reddy, V. V. V. N. S. R. Rao, S. Ravikanth, B. Narsaiah, P. S. Rao, K. Ravikumar and B. Sridhar, Tetrahedron, 2005, 61, 3999 CrossRef CAS; (b) N. G. Andersen, S. P. Maddaford and B. A. Keay, J. Org. Chem., 1996, 61, 9556 CrossRef CAS.
- (a) T. M. Cardozo and M. A. C. Nascimento, J. Mater. Sci., 2005, 40, 3549 CrossRef CAS; (b) D. R. Kanis, M. A. Ratner and T. J. Marks, Chem. Rev., 1994, 94, 195 CrossRef CAS.
- (a) R. L. Carroll and C. B. Gorman, Angew. Chem., Int. Ed., 2002, 41, 4378 CrossRef; (b) M. Bendikov, F. Wudl and D. F. Perepichka, Chem. Rev., 2004, 104, 4891 CrossRef CAS.
- (a) G. Olah, Friedel–Crafts and Related Reactions, Wiley Interscience, New York, 1963, vol. I–IV Search PubMed; (b) D. E. Pearson and C. A. Buehler, Synthesis, 1972, 533 CrossRef CAS; (c) R. Taylor, Electrophilic Aromatic Substitution, Wiley, NewYork, 1990 Search PubMed.
- (a) M. B. Smith and J. March, Advanced Organic Chemistry, Wiley-Interscience, New York, 5th edn, 2001, ch. 13, p. 850 Search PubMed; (b) J. A. Zoltewicz, Top. Curr. Chem., 1975, 59, 33 CrossRef CAS; (c) E. Buncel, J. M. Dust and F. Terrier, Chem. Rev., 1995, 95, 2261 CrossRef CAS.
- (a) G. Dyker, Angew. Chem., Int. Ed., 1999, 38, 1698 CrossRef; (b) J. Hassan, M. Se'vignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS.
- (a) V. Snieckus, Chem. Rev., 1990, 90, 879 CrossRef CAS; (b) W. W. Lin, O. Baron and P. Knochel, Org. Lett., 2006, 8, 5673 CrossRef CAS.
- (a) M. Adib, B. Mohammadi, M. Mahdavi, A. Abbasi and M. Riazati Kesheh, Synlett, 2007, 2497 CrossRef CAS.
- (a) K. H. Dotz and P. Tomuschat, Chem. Soc. Rev., 1999, 28, 187 RSC; (b) H. Wang, J. Huang, W. D. Wulff and A. L. Rheingold, J. Am. Chem. Soc., 2003, 125, 8980 CrossRef CAS; (c) A. V. Vorogushin, W. D. Wulff and H.-J. Hansen, J. Am. Chem. Soc., 2002, 124, 6512 CrossRef CAS.
- (a) Z. Xi, K. Sato, Y. Gao, J. Lu and T. Takahashi, J. Am. Chem. Soc., 2003, 125, 9568 CrossRef CAS; (b) T. Takahashi, M. Ishikawa and S. Huo, J. Am. Chem. Soc., 2002, 124, 388 CrossRef CAS.
- (a) R. L. Danheiser, R. G. Brisbois, J. J. Kowalczyk and R. F. Miller, J. Am. Chem. Soc., 1990, 112, 3093 CrossRef CAS; (b) R. L. Danheiser and S. K. Gee, J. Org. Chem., 1984, 49, 1672 CrossRef CAS.
- (a) L. V. R. Bonaga, H.-C. Zhang, A. F. Moretto, H. Ye, D. A. Gauthier, J. Li, G. C. Leo and B. E. Maryanoff, J. Am. Chem. Soc., 2005, 127, 3473 CrossRef CAS.
- (a) N. Asao, T. Nogami, S. Lee and Y. Yamamoto, J. Am. Chem. Soc., 2003, 125, 10921 CrossRef CAS; (b) N. Asao, K. Takahashi, S. Lee, T. Kasahara and Y. Yamamoto, J. Am. Chem. Soc., 2002, 124, 12650 CrossRef CAS; (c) N. Asao, H. Aikawa and Y. Yamamoto, J. Am. Chem. Soc., 2004, 126, 7458 CrossRef CAS.
- (a) P. Langer and G. Bose, Angew. Chem., Int. Ed., 2003, 42, 4033 CrossRef CAS; (b) A. R. Katritzky, J. Li and L. Xie, Tetrahedron, 1999, 55, 8263 CrossRef CAS.
- (a) S. Serra, C. Fuganti and A. Moro, J. Org. Chem., 2001, 66, 7883 CrossRef CAS; (b) P. Turnbull and H. W. Moore, J. Org. Chem., 1995, 60, 644 CrossRef CAS.
- N. A. Anisimova, A. A. Kuzhaeva, G. A. Berkova and V. M. Berestovitskaya, Russ. J. Org. Chem., 2011, 81, 1845 CrossRef CAS.
- M. Adib, B. Mohammadi, S. Ansari, H. R. Bijanzadeh and L.-G. Zhu, Synthesis, 2010, 1526 CrossRef CAS.
- D. Xue, J. Li, Z.-T. Zhang and J.-G. Deng, J. Org. Chem., 2007, 72, 5443 CrossRef CAS.
- W. Su, K. Ding and Z. Chen, Tetrahedron Lett., 2009, 50, 636 CrossRef CAS.
- K. U. Sadek, R. M. Shaker, M. A. Elrady and M. H. Elnagdi, Tetrahedron Lett., 2010, 51, 6319 CrossRef CAS.
- S. Gabrielli, A. Palmieri, D. S. Panmand, D. Lanari, L. Vaccaro and R. Ballini, Tetrahedron, 2012, 68, 8231 CrossRef CAS.
- P. Li, L.-L. Luo, X.-S. Li and J.-W. Xie, Tetrahedron, 2010, 66, 7590 CrossRef CAS.
- V. Y. Korotaev, A. Y. Barkov and V. Y. Sosnovskikh, Tetrahedron, 2013, 69, 9642 CrossRef CAS.
- A. Riahi, M. Shkoor, R. A. Khera, H. Reinke and P. Langer, Tetrahedron Lett., 2009, 50, 3017 CrossRef CAS.
- G. Dupeyre, P. Lemoine, N. Ainseba, S. Michel and X. Cachet, Org. Biomol. Chem., 2011, 9, 7780 CAS.
- (a) H. D. King, D. J. Denhart, J. A. Deskus, J. L. Ditta, J. R. Epperson, M. A. Higgins, J. E. Kung, L. R. Marcin, C. P. Sloan, G. K. Mattson, T. F. Molski, R. G. Krause, R. L. Bertekap, N. J. Lodge, R. J. Mattson and J. E. Macor, Bioorg. Med. Chem. Lett., 2007, 17, 5647 CrossRef CAS; (b) K. Saeki, T. Matsuda, T. Kato, K. Yamada, T. Mizutani, S. Matsui, K. Fukuhara and N. Miyata, Biol. Pharm. Bull., 2003, 26, 448 CrossRef CAS; (c) R. W. Hartmann, A. Palusczak, F. Lacan, G. Ricci and R. Ruzziconi, J. Enzyme Inhib. Med. Chem., 2004, 19, 145 CrossRef CAS; (d) O. Silva and E. T. Gomes, J. Nat. Prod., 2003, 66, 447 CrossRef CAS; (e) J. Dai, Y. Liu, Y.-D. Zhou and D. G. Nagle, J. Nat. Prod., 2007, 70, 1824 CrossRef CAS; (f) X. Xie and M. C. Kozlowski, Org. Lett., 2001, 3, 2661 CrossRef CAS; (g) T. Ukita, Y. Nakamura, A. Kubo, Y. Yamamoto, M. Takahashi, J. Kotera and T. Ikeo, J. Med. Chem., 1999, 42, 1293 CrossRef CAS; (h) R. S. Ward, Nat. Prod. Rep., 1995, 12, 183 RSC.
- M. R. Boyd, Y. F. Hallock, J. H. Cardellina II, K. P. Manfredi, J. W. Blunt, J. B. McMahon, R. W. Buckheit Jr, G. Bringmann, M. Schaffer, G. M. Cragg, D. W. Thomas and J. G. Jato, J. Med. Chem., 1994, 37, 1740 CrossRef CAS.
- (a) Y. F. Hallock, K. P. Manfredi, J. W. Blunt, J. H. Cardellina II, M. Schaffer, K. P. Gluden, G. Bringmann, A. Y. Lee, J. Clardy, G. Francois and M. R. Boyd, J. Org. Chem., 1994, 59, 6349 CrossRef CAS; (b) T. R. Hoye and L. Mi, Tetrahedron Lett., 1996, 37, 3097 CrossRef CAS; (c) T. R. Hoye and L. Mi, J. Org. Chem., 1997, 62, 8586 CrossRef CAS.
- A. I. Meyers and J. Willemsen, Chem. Commun., 1997, 1573 RSC.
- J. M. Trujillo, R. E. Jorge, E. Navarro and J. Boada, Phytochemistry, 1990, 29, 2991 CrossRef CAS.
- (a) M. Berthod, G. Mignani, G. Woodward and M. Lemaire, Chem. Rev., 2005, 105, 1801 CrossRef CAS; (b) H.-L. Teng, F.-L. Luo, H.-Y. Tao and C.-J. Wang, Org. Lett., 2011, 13, 5600 CrossRef CAS.
- C. B. de Koning, A. L. Rousseau and W. A. L. van Otterlo, Tetrahedron, 2003, 59, 7 CrossRef CAS ; and the references cited therein.
- (a) J. L. Charlton, C. J. Oleschuk and G. L. Chee, J. Org. Chem., 1996, 61, 3452 CrossRef CAS; (b) T. Kitamura, M. Yamane, K. Inoue, M. Todaka, N. Fukatsu, Z. Meng and Y. Fujiwara, J. Am. Chem. Soc., 1999, 121, 11674 CrossRef CAS.
- S. Saito and Y. Yamamoto, Chem. Rev., 2000, 100, 2901 CrossRef CAS.
- (a) N. Iwasawa, M. Shido, K. Maeyama and H. Kusama, J. Am. Chem. Soc., 2000, 122, 10226 CrossRef CAS; (b) K. Maeyama and N. Iwasawa, J. Am. Chem. Soc., 1998, 120, 1928 CrossRef CAS; (c) K. Maeyama and N. Iwasawa, J. Org. Chem., 1999, 64, 1344 CrossRef CAS.
- (a) K.-S. Huang and E.-C. Wang, Tetrahedron Lett., 2001, 42, 6155 CrossRef CAS; (b) P. Evans, R. Grigg, M. I. Ramzan, V. Sridharan and M. York, Tetrahedron Lett., 1999, 40, 3021 CrossRef CAS.
- (a) R. Tiedemann, P. Turnbull and H. W. Moore, J. Org. Chem., 1999, 64, 4030 CrossRef CAS; (b) T. Matsumoto, T. Hamura, M. Miyamoto and K. Suzuki, Tetrahedron Lett., 1998, 39, 4853 CrossRef CAS.
- (a) L. F. Somerville and C. F. H. Allen, Organic Syntheses, ed. A. H. Blatt, Wiely, New York, 1943, vol. 2, p. 81 Search PubMed; (b) J. Wennerburg, C. Olofsson and T. Frejd, J. Org. Chem., 1998, 63, 3595 CrossRef.
- (a) D. C. Harrowven, M. Bradley, J. L. Castro and S. R. Flanagan, Tetrahedron Lett., 2001, 42, 6973 CrossRef CAS; (b) R. A. Aitken, C. Boeters and J. J. Morrison, J. Chem. Soc., Perkin Trans. 1, 1997, 2625 RSC.
- (a) E. Hasegawa, Y. Tamura and E. Tosaka, Chem. Commun., 1997, 1895 RSC; (b) F. B. Mallory and C. W. Mallory, Photocyclization of Stilbenes and Related Molecules. In Organic Reactions, Wiley, New York, 1984, vol. 30, p. 1 Search PubMed.
- (a) C. Ikemoto, T. Kawano and I. Ueda, Tetrahedron Lett., 1998, 39, 5053 CrossRef CAS; (b) N. Choy, C.-S. Kim, C. Ballestero, L. Artigas, C. Diez, F. Lichtenberger, J. Shapiro and K. C. Russell, Tetrahedron Lett., 2000, 41, 6955 CrossRef CAS.
- S.-G. Li, X.-Q. Hu, Z.-X. Jia and P.-F. Xu, Tetrahedron, 2010, 66, 8557 CrossRef CAS.
- C. Keene and L. Kürti, Synthesis, 2013, 1719 CAS.
| This journal is © The Royal Society of Chemistry 2014 |