Synthesis of a landomycinone skeleton via Masamune–Bergmann cyclization†
Abstract
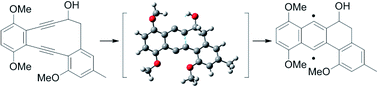
In this report, a synthetic study of landomycinone via Masamune–Bergmann cyclization is described. A 10-membered 1,2-dialkynylbenzene derivative was designated as a key intermediate in the formation of an angular tetracyclic core via Masamune–Bergmann cyclization. Cyclization was expected to proceed under mild heating conditions based on a DFT transition state analysis of the 10-membered enediyne. The enediyne was successfully prepared by intramolecular NHK cyclization in good yield and underwent Masamune–Bergman cyclization at 70 °C for 2 h. However, an undesired β-elimination of the secondary alcohol was involved in the cyclization. In addition, iodination at the 12 position did not occur due to the steric hindrance of two methyl groups. This methodology should be widely applicable to the synthesis of various types of highly oxy-functionalized anthraquinone derivatives as well as landomycinone, and should be a useful way to clarify structure–activity relationships.

 Please wait while we load your content...
Please wait while we load your content...