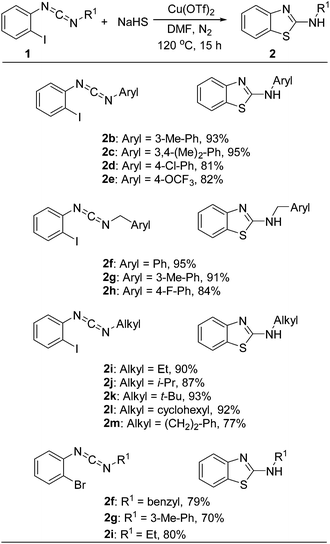
Copper-catalyzed synthesis of 2-aminobenzothiazoles from carbodiimide and sodium hydrosulfide†
Weilan Zenga,
Pan Danga,
Xiaoyun Zhanga,
Yun Liang*a and
Caiyun Peng*b
aNational & Local Joint Engineering Laboratory for New Petro-chemical Materials and Fine Utilization of Resources, Key Laboratory of Chemical Biology and Traditional Chinese Medicine Research, Ministry of Education, Hunan Normal University, Changsha, Hunan 410081, China. E-mail: yliang@hunnu.edu.cn; paudy@126.com; Fax: +86 0731 88872533
bSchool of Pharmaceutical Science, Hunan University of Chinese Medicine, Changsha, Hunan 410208, China
First published on 14th July 2014
Abstract
An efficient copper-catalyzed method for the synthesis of a variety of 2-aminobenzothiazoles has been developed. The reaction proceeded from carbodiimide and sodium hydrosulfide via a tandem reaction in the presence of copper(II) trifluoromethanesulfonate to afford the corresponding 2-aminobenzothiazole derivatives in good to perfect yields.
2-Aminobenzothiazoles,1–5 categorized as significant derivatives of benzothiazoles, are broadly found in bioorganic and medicinal chemistry with applications in drug discovery and development for the treatment of various diseases, such as AIDS,2 diabetes,3 epilepsy,4 and tuberculosis.5 Consequently, many efficient methods were developed for the synthesis of 2-aminobenzothiazoles. Among them, the common method was based on metal-catalyzed intermolecular cross-coupling reaction between 2-halobenzothiazoles and amines,6 2-aminobenzothiazoles and aryl halides,7 or simple benzothiazoles and amines.8 The other two methods of direct construction of 2-aminobenzothiazole have attracted more attention from the viewpoints of operational simplicity: (1) intramolecular cyclization of o-haloarylthioureas or arylthioureas;9 and (2) intermolecular cyclization of 2-halophenylamines or 2-aminobenzenethiols with isothiocyanates.10 However, these methods usually require several steps and harsh reaction conditions for the preparation of sulfur-containing substrates such as benzothiazoles, arylthioureas, isothiocyanates, which limit their application in synthesis. Recently, Ma and co-worker developed a simple method for the synthesis of 2-aminobenzothiazoles used the carbon disulfide as sulfur source.11 Nevertheless, the toxicity and unpleasant odor of carbon disulfide impedes its application. Therefore, used simple, nontoxic, readily available sulfur sources for the synthesis of 2-aminobenzothiazoles are of great value.
Recently, the nontoxic, odorless, and readily available sulfur sources such as metal sulfides have received considerable attention to synthesize the sulfur-containing heterocyclic compounds via a double thiolation reaction.12 We are also interested in this synthetic strategy, and have successfully synthesized benzo[b]thiophene,13 benzo[d]thiazole14 and benzo[d]thiazol-2(3H)-one15 used potassium sulfide as sulfur source. In the present research, we found that o-haloarylcarboniimide16 and metal sulfides could undergo a cascade process to afford 2-aminobenzothiazoles. As shown in Scheme 1, the proposed reaction might proceed through a cross-coupling of o-haloarylcarboniimide 1 and NaHS under copper-catalyzed conditions (step a, the plausible intermediate 3 would be formed, and copper catalyst was regenerated),12–15,17 followed by the formation of 2-aminobenzothiazoles 2 or the intermediate of benzo[d]thiazol-2(3H)-imine 4 via an intramolecular nucleophile addition (step b, the process might be analogous to those reported nucleophile addition to a certain extent).16,18 Rearrangement and isomerization of the intermediate 4 also give rise to the product 2 (step c). Here in, we wish to detail our results.
 | ||
| Scheme 1 Proposed one-pot synthesis of 2-aminobenzothiazoles via a copper-catalyzed coupling/addition process. | ||
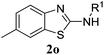
In this work, N-phenylbenzo[d]thiazol-2-amine was obtained in good yields from N-(2-iodophenyl)-N-phenylmethanediimine 1a and NaHS in one pot, via a copper-catalyzed double thiolation. The results of the screening for optimal reaction conditions are shown in Table 1. Our investigation started by an attempted thiolation of substrate 1a with K2S in DMF at 120 °C in the presence of CuBr2 as the catalyst, and the desired product 2a was isolated in 48% yield (entry 1). This result encouraged us to develop an efficient system to synthesize 2-aminobenzothiazole using N-(2-iodophenyl)-carbodiimine as a starting substrate. A variety of sulfur sources, such as K2S, Na2S, NaHS, S, NaS2O3, were screened (entries 1–5). The results indicated that NaHS was the best one for this reaction. Subsequently, the effects of copper catalysts (including CuBr, CuI, CuCl, CuCN, CuOTf, Cu(OAc)2, Cu(OTf)2) are examined (entries 6–12). Cu(OTf)2 achieved the best result, and the product 2a was obtained in 74% yield. It is noteworthy that copper II has much better catalysis activity than copper I. The possible reason attributes copper II could promote the nucleophile addition of carboniimide.18 Without the copper catalyst, the desired product decreased to 11% yield (entry 13). Then, the effects of ligands (include 2,2′-bipyridine, 1,10-phen, L(−)-proline, TMEDA) were checked also (entries 14–17). However, the ligands did not show better results. Solvents such as DMSO and NMP were evaluated, and 60% and 67% yield of the product 2a were isolated respectively (entries 18–19). Finally, the amount of catalyst and the reaction temperature were evaluated, and relatively low yields were found with any reduction in the reaction temperature or the amount catalyst (entries 20–21). Thus, the optimized reaction condition were as follow: 1a (0.3 mmol), NaHS (0.9 mmol), Cu(OTf)2 (20 mol%), in DMF (2 mL) under a N2 atmosphere at 120 °C.
| Entry | Sulfur source | Catalyst | Ligand | Solvent | Yieldb/2a |
|---|---|---|---|---|---|
| a Conditions: 1a (0.30 mmol), sufur source (0.90 mmol), Cu catalyst (20 mol%), ligand (20 mol%), solvent (2 mL), N2, 120 °C, 15 h.b Isolated yield.c Cs2CO3 (0.90 mmol).d 100 °C.e Cu(OTf)2 (10 mol%). | |||||
| 1 | K2S | CuBr2 | — | DMF | 48 |
| 2 | NaHS | CuBr2 | — | DMF | 66 |
| 3 | Na2S | CuBr2 | — | DMF | 53 |
| 4 | Na2S2O3 | CuBr2 | — | DMF | 58 |
| 5c | S | CuBr2 | — | DMF | 36 |
| 6 | NaHS | CuI | — | DMF | 46 |
| 7 | NaHS | CuBr | — | DMF | 53 |
| 8 | NaHS | CuCl | — | DMF | 41 |
| 9 | NaHS | CuCN | — | DMF | 45 |
| 10 | NaHS | CuOTf | — | DMF | 48 |
| 11 | NaHS | Cu(OAc)2 | — | DMF | 60 |
| 12 | NaHS | Cu(OTf)2 | — | DMF | 74 |
| 13 | NaHS | — | — | DMF | 11 |
| 14 | NaHS | Cu(OTf)2 | 1,10-Phen | DMF | 71 |
| 15 | NaHS | Cu(OTf)2 | 2,2-Py | DMF | 62 |
| 16 | NaHS | Cu(OTf)2 | TMEDA | DMF | 68 |
| 17 | NaHS | Cu(OTf)2 | L(−)-Proline | DMF | 73 |
| 18 | NaHS | Cu(OTf)2 | — | DMSO | 60 |
| 19 | NaHS | Cu(OTf)2 | — | NMP | 67 |
| 20d | NaHS | Cu(OTf)2 | — | DMF | 70 |
| 21e | NaHS | Cu(OTf)2 | — | DMF | 64 |
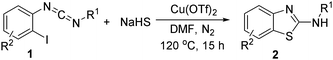
Under the optimized conditions, the substituent of the nitrogen moiety of o-iodobenzylcarboniimide was screened, and the results were summarized in Table 2. Various N-substituted 2-aminobenzothiazoles were obtained from good to perfect yields. Initially, the substituents of aryl were screened. The results showed that increasing the electron density on the nonhalogenated ring might favor the intramolecular addition process. For instance, the presence of a weak electron-donating group (m-Me) and a weak electron-withdrawing group (p-Cl) on the aromatic ring of 1 provided 93% and 81% yield of corresponding products. Similarly, N-benzyl substituted 2-aminobenzothiazoles could be obtained in good to high isolated yields. For example, N-benzylbenzo[d]thiazol-2-amine was obtained in 95% yield under the optimized condition. For the N-alkyl substituted benzo[d]thiazol-2-amine, they with linear-chain, branched-chain, and cycloalkyl groups could all be afforded in perfect yields. This result showed that the alkyl substituent did not remarkably affect the reaction. Finally, we investigated the reactivity of o-bromobenzylcarboniimides. Importantly, the o-bromobenzylcarboniimides could efficiently reacted with NaHS and good yields of the products were given.
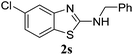
To expand the scope of this methodology, we also examined a series of substituted N-benzyl-N-(2-iodophenyl)methanediimine and N-(4-chlorophenyl)-N-(2-iodophenyl)methanediimine. As summarized in Table 3, for N-benzyl-N-(2-iodophenyl)methanediimine with either electron-withdrawing groups such as chloro (4-Cl, 5-Cl) and trifluoromethyl or electron-donating group such as methyl (4-methyl, 4,6-dimethyl) on iodobenzene ring, all well-tolerated under the reaction conditions and proceed with almost equal efficiency. These results indicated that electronic effect on benzene ring did not play a significant role in regulating the reaction, and revealed the inherent high reactivity of o-iodobenzylcarboniimide. Unfortunately, bromo-substituted 2-aminobenzothiazole only afforded in 66% yield. However, bromo-substituted 2-aminobenzothiazole could offer an opportunity for further cross-coupling, and facilitating the expedient synthesis of complex compounds.
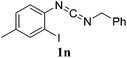
To our delight, this synthetic method to synthesize 2-aminobenzithiazoles could be further extend from the initial starting material in one step. For example, N-(2-iodophenyl)triphenyliminophosphrane reacted with isocyanate in DMF for 12 h, then NaHS and Cu(OTf)2 were added, and the reaction was further stirred for 15 h at 120 °C, and the corresponding 2-aminobenzithiazoles were obtained in good yields (Scheme 2).
In summary, we have developed an efficient coupling/addition tandem reaction from o-haloarylcarboniimide and NaHS for the synthesis of 2-aminobenzothiazoles. In this copper-catalyzed system, the tolerance of diverse functional groups in o-haloarylcarboniimide makes this present system attractive in the synthesis of various 2-aminobenzothiazoles. To our best knowledge, this is the first example of the use of NaHS as the sulfur source in the synthesis of 2-aminobenzothiazole derivatives.
Acknowledgements
This work was supported by the Natural Science Foundation of China (21072054), the Research Project of Chinese Ministry of Education (213027A, 20094306120003), Hunan Provincial Natural Science Foundation (12JJ2009), Scientific Research Fund of Hunan Provincial Education Department (12A095) and Aid Program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province for financial support.Notes and references
- For selected examples, see: (a) S. Bondock, W. Fadaly and M. A. Metwally, Eur. J. Med. Chem., 2010, 45, 3692 CrossRef CAS PubMed; (b) R. D. Carpenter, M. Andrei, O. H. Aina, E. Y. Lau, F. C. Lightstone, R. Liu, K. S. Lam and M. J. Kurth, J. Med. Chem., 2009, 52, 14 CrossRef CAS PubMed; (c) A. D. Jordan, C. Luo and A. B. Reitz, J. Org. Chem., 2003, 68, 8693 CrossRef CAS PubMed; (d) A. R. Katritzky, D. O. Tymoshenko, D. Monteux, V. Vvedensky, G. Nikonov, C. B. Cooper and M. Deshpande, J. Org. Chem., 2000, 65, 8059 CrossRef CAS PubMed.
- S. Massari, D. Daelemans, M. L. Barreca, A. Knezevich, S. Sabatini, V. Cecchetti, A. Marcello, C. Pannecouque and O. Tabarrini, J. Med. Chem., 2010, 53, 641 CrossRef CAS PubMed.
- H. Suter and H. Zutter, Helv. Chim. Acta, 1967, 50, 1084 CrossRef CAS PubMed.
- S. J. Hays, M. J. Rice, D. F. Ortwine, G. Johnson, R. D. Schwartz, D. K. Boyd, L. F. Copeland, M. G. Vartanian and P. A. Boxer, J. Pharm. Sci., 1994, 83, 1425 CrossRef CAS.
- V. G. Shirke, A. S. Bobade, R. P. Bhamaria, B. G. Khadse and S. R. Sengupta, Indian Drugs, 1990, 27, 350 CAS.
- (a) S. Toulot, T. Heinrich and F. R. Leroux, Adv. Synth. Catal., 2013, 355, 3263 CrossRef CAS; (b) M. D. Charles, P. Schultz and S. L. Buchwald, Org. Lett., 2005, 7, 3965 CrossRef CAS PubMed; (c) M. W. Hooper, M. Utsunomiya and J. F. Hartwig, J. Org. Chem., 2003, 68, 2861 CrossRef CAS PubMed.
- (a) S. N. Murthy, B. Madhav, V. P. Reddy and Y. V. D. Nageswara, Adv. Synth. Catal., 2010, 352, 3241 CrossRef CAS; (b) Q. Shen, T. Ogata and J. F. Hartwig, J. Am. Chem. Soc., 2008, 130, 6586 CrossRef CAS PubMed; (c) A. Miloudi, D. EI-Abed, G. Boyer, J. P. Finet, J. P. Galy and D. Siri, Eur. J. Org. Chem., 2004, 1509 CrossRef CAS; (d) J. Yin, M. M. Zhao, M. A. Huffman and J. M. McNamara, Org. Lett., 2002, 4, 3481 CrossRef CAS PubMed.
- (a) A. Armstrong and J. C. Collins, Angew. Chem., Int. Ed., 2010, 49, 2282 CrossRef CAS PubMed; (b) D. Monguchi, T. Fujiwara, H. Furukawa and A. Mori, Org. Lett., 2009, 11, 1607 CrossRef CAS PubMed; (c) Q. Wang and S. L. Schreiber, Org. Lett., 2009, 11, 5178 CrossRef CAS PubMed; (d) S. H. Cho, J. Y. Kim, S. Y. Lee and S. Chang, Angew. Chem., Int. Ed., 2009, 48, 9127 CrossRef CAS PubMed.
- (a) S.-G. Kim, S.-L. Jung, G.-H. Lee and Y.-D. Gong, ACS Comb. Sci., 2013, 15, 29 CrossRef CAS PubMed; (b) P. Saha, T. Ramana, N. Purkait, M. A. Ali, R. Paul and T. Punniyamurthy, J. Org. Chem., 2009, 74, 8719 CrossRef CAS PubMed; (c) K. Inamoto, C. Hasegawa, K. Hiroya and T. Doi, Org. Lett., 2008, 10, 5147 CrossRef CAS PubMed; (d) J. Wang, F. Peng, J.-L. Jiang, Z.-J. Lu, L.-Y. Wang, J. Bai and Y. Pan, Tetrahedron Lett., 2008, 49, 467 CrossRef CAS PubMed; (e) L. L. Joyce, G. Evindar and R. A. Batey, Chem. Commun., 2004, 446 RSC; (f) C. Benedí, F. Bravo, P. Uriz, E. Fernández, C. Claver and S. Castillón, Tetrahedron Lett., 2003, 44, 6073 CrossRef.
- (a) R. Yao, H. Liu, Y. Wu and M. Cai, Appl. Organomet. Chem., 2013, 27, 109 CrossRef CAS; (b) W. Zhang, Y. Yue, D. Yu, L. Song, Y. Y. Xu, Y. J. Tian and Y. J. Guo, Adv. Synth. Catal., 2012, 354, 2283 CrossRef CAS; (c) Y. J. Guo, R. Y. Tang, P. Zhong and J. H. Li, Tetrahedron Lett., 2010, 51, 649 CrossRef CAS PubMed; (d) J. W. Qiu, X. G. Zhang, R. Y. Tang, P. Zhong and J. H. Li, Adv. Synth. Catal., 2009, 351, 2319 CrossRef CAS; (e) G. Shen, X. Lv and W. Bao, Eur. J. Org. Chem., 2009, 5897 CrossRef CAS; (f) Q. Ding, X. He and J. Wu, J. Comb. Chem., 2009, 11, 587 CrossRef CAS PubMed.
- D. Ma, X. Lu, L. Shi, H. Zhang, Y. Jiang and X. Liu, Angew. Chem., Int. Ed., 2011, 50, 1118 CrossRef CAS PubMed.
- (a) Z. Qiao, H. Liu, X. Xiao, Y. Fu, J. Wei, Y. Li and X. Jiang, Org. Lett., 2013, 15, 2594 CrossRef CAS PubMed; (b) J. Li, Y. Zhang, Y. Jiang and D. Ma, Tetrahedron Lett., 2012, 53, 2511 CrossRef CAS PubMed; (c) L.-L. Sun, C.-L. Deng, R.-Y. Tang and X.-G. Zhang, J. Org. Chem., 2011, 76, 7546 CrossRef CAS PubMed; (d) W. You, X. Yan, Q. Liao and C. Xi, Org. Lett., 2010, 12, 3930 CrossRef CAS PubMed; (e) C.-L. Li, X.-G. Zhang, R.-Y. Tang, P. Zhong and J.-H. Li, J. Org. Chem., 2010, 75, 7037 CrossRef CAS PubMed; (f) D. Ma, S. Xie, P. Xue, X. Zhang, J. Dong and Y. Jiang, Angew. Chem., Int. Ed., 2009, 48, 4222 CrossRef CAS PubMed.
- X. Zhang, W. Zeng, Y. Yang, H. Huang and Y. Liang, Synlett, 2013, 24, 1693 CrossRef PubMed.
- X. Zhang, W. Zeng, Y. Yang, H. Huang and Y. Liang, Org. Lett., 2014, 16, 876 CrossRef CAS PubMed.
- Y. Yang, X. Zhang, W. Zeng, H. Huang and Y. Liang, RSC Adv., 2014, 4, 6090 RSC.
- (a) G. Qiu, Y. Lu and J. Wu, Org. Biomol. Chem., 2013, 11, 798 RSC; (b) G. Qiua and J. Wu, Chem. Commun., 2012, 48, 6046 RSC; (c) B. Roberts, D. Liptrot, T. Luker, M. J. Stocks, C. Barber, N. Webb, R. Dods and B. Martin, Tetrahedron Lett., 2011, 52, 3793 CrossRef CAS PubMed; (d) F. Zeng and H. Alper, Org. Lett., 2010, 12, 1188 CAS.
- (a) R. Cano, D. J. Ramon and M. Yus, J. Org. Chem., 2011, 76, 654 CrossRef CAS PubMed; (b) Y. Jiang, Y. Qin, S. Xie, X. Zhang, J. Dong and D. Ma, Org. Lett., 2009, 11, 5250 CrossRef CAS PubMed.
- (a) F. Zhao, Y. Wang, W.-X. Zhang and Z. Xi, Org. Biomol. Chem., 2012, 10, 6266 RSC; (b) F. Wang, S. Cai, Q. Liao and C. Xi, J. Org. Chem., 2011, 76, 3174 CrossRef CAS PubMed; (c) D. Li, J. Guang, W.-X. Zhang, Y. Wang and Z. Xi, Org. Biomol. Chem., 2010, 8, 1816 RSC; (d) X. Lv and W. Bao, J. Org. Chem., 2009, 74, 5618 CrossRef CAS PubMed; (e) Z. Wang, Y. Wang, W.-X. Zhang, Z. Hou and Z. Xi, J. Am. Chem. Soc., 2009, 131, 15108 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra04047c |
| This journal is © The Royal Society of Chemistry 2014 |