Iron-catalysed tandem isomerisation/hydrosilylation reaction of allylic alcohols with amines†
Abstract
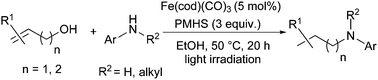
An iron(0)-catalysed cascade synthesis of N-alkylated anilines from allylic or homoallylic alcohols and primary and secondary anilines under hydrosilylation conditions has been developed. Notably, a simple Fe(cod)(CO)3 complex (cod = cycloocta-1,5-diene) was used as a precatalyst under visible light activation in ethanol in the presence of the inexpensive and non-toxic PMHS (polymethylhydrosiloxane) as the hydrosilane source. This methodology is based on a three step-one sequence process involving isomerisation of allylic alcohols/condensation with anilines/reduction reactions.

 Please wait while we load your content...
Please wait while we load your content...