Functionalized mesoporous carbon nanoparticles for targeted chemo-photothermal therapy of cancer cells under near-infrared irradiation
Abstract
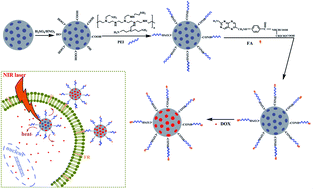
Chemo-photothermal therapy with the combination of chemotherapy and photothermal therapy has emerged as a promising anticancer treatment for its synergistic effects. In this work, the functionalized mesoporous carbon nanoparticles (FA/PEI/O-MCN) were constructed by modifying the mesoporous carbon nanoparticles (MCN) with polyethylenimine (PEI) and folic acid (FA) for the targeted chemo-photothermal therapy. The FA/PEI/O-MCN exhibited strong light absorption and high photothermal conversion efficiency in the near-infrared (NIR) region due to the graphitic structure of MCN. Meanwhile, FA/PEI/O-MCN displayed high drug loading capacity using doxorubicin hydrochloride (DOX) as a model drug. Flow cytometry analysis and competitive binding experiments verified that the FA modification could significantly enhance the uptake of FA/PEI/O-MCN by HeLa cells with folate receptors (FR) over-expressing. Comparing with chemotherapy or photothermal therapy alone, the DOX-loaded FA/PEI/O-MCN demonstrated the synergistic effects and resulted in the higher therapeutic efficacy. We believe that the FA/PEI/O-MCN could be applied as an efficient chemo-photothermal platform to realize the targeted synergistic therapy.

 Please wait while we load your content...
Please wait while we load your content...